Osimertinib for Front-Line Treatment of Locally Advanced or Metastatic EGFR-Mutant NSCLC Patients: Efficacy, Acquired Resistance and Perspectives for Subsequent Treatments
- PMID: 33324104
- PMCID: PMC7733376
- DOI: 10.2147/CMAR.S218751
Osimertinib for Front-Line Treatment of Locally Advanced or Metastatic EGFR-Mutant NSCLC Patients: Efficacy, Acquired Resistance and Perspectives for Subsequent Treatments
Abstract
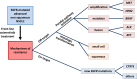
Non-small cell lung cancer (NSCLC) is one of the most efficient models for precision medicine in oncology. The most appropriate therapeutic for the patient is chosen according to the molecular characteristics of the tumor, schematically distributed between immunogenicity and oncogenic addiction. For this last concept, advanced NSCLC with epidermal growth factor receptor (EGFR) mutation is one of the most illustrative models. EGFR-tyrosine kinase inhibitors (TKIs) are the therapeutic backbone for this type of tumor. The recent development of a third-generation TKI, osimertinib, has been a new step forward in the treatment of NSCLC patients. In this article, we first review the clinical development of osimertinib and highlight its efficacy results. We then present the most frequent tumor escape mechanisms when osimertinib is prescribed in first line: off-target (MET amplification, HER2 amplification, BRAF mutation, gene fusions, histologic transformation) and on-target mechanisms (EGFR mutation). Finally, we discuss subsequent biomarker-driven treatment strategies.
Keywords: EGFR mutation; acquired resistance; lung cancer; targeted therapy.
© 2020 Denis and Bennouna.
Conflict of interest statement
Professor Marc G Denis reports grants and personal fees from Boehringer Ingelheim and personal fees from Astra Zeneca, during the conduct of the study; and grants and personal fees from Takeda, and personal fees from Roche Diagnostics and BMS, outside the submitted work. Professor Jaafar Bennouna reports grants and personal fees from Astra Zeneca, during the conduct of the study; and personal fees from BMS, Roche, MSD, and Servier, outside the submitted work. The authors report no other potential conflicts of interest for this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous