Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, proBDNF
- PMID: 33324399
- PMCID: PMC7723927
- DOI: 10.3389/fimmu.2020.575607
Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, proBDNF
Abstract
Background: Brain-derived neurotrophic factor (BDNF) plays a role in synaptic plasticity and neuroprotection. BDNF has well-established pro-survival effects, whereas its precursor protein, proBDNF, induces apoptosis. Thus, it has been suggested that the proBDNF/BDNF ratio could be an indicator of neuronal health. Access to neurons is, understandably, limited. Because of their similarities, platelets have been put forward as a non-invasive biomarker of neuronal health; indeed, they store large quantities of BDNF and can release it into circulation upon activation, similarly to neurons. However, whether platelets also express the precursor proBDNF protein remains unknown. We therefore sought to characterize proBDNF levels in human platelets and plasma.
Methods: The presence of proBDNF was assessed by immunoblotting, cell fractionation, flow cytometry, and confocal microscopy in washed platelets from 10 healthy volunteers. Platelets from 20 independent healthy volunteers were activated with several classical agonists and the release of BDNF and proBDNF into plasma was quantified by ELISA.
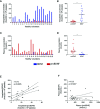
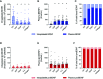
Results: Platelets expressed detectable levels of proBDNF (21 ± 13 fmol/250 x 106 platelets). ProBDNF expression was mainly localized in the intracellular compartment. The proBDNF to BDNF molar ratio was ~1:5 in platelets and 10:1 in plasma. In stark contrast to the release of BDNF during platelet activation, intraplatelet and plasma concentrations of proBDNF remained stable following stimulation with classical platelet agonists, consistent with non-granular expression.
Conclusions: Platelets express both the mature and the precursor form of BDNF. Whether the intraplatelet proBDNF to BDNF ratio could be used as a non-invasive biomarker of cognitive health warrants further investigation.
Keywords: brain-derived neurotrophic factor; plasma; platelets; pro-BDNF; secretion.
Copyright © 2020 Le Blanc, Fleury, Boukhatem, Bélanger, Welman and Lordkipanidzé.
Conflict of interest statement
ML has received speaker fees from Bayer; has participated in industry-funded trials from Idorsia; has served on advisory boards for Servier; and has received in-kind and financial support for investigator-initiated grants from Leo Pharma, Roche Diagnostics, Aggredyne, and Fujimori Kogyo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Borodinova AA, Salozhin SV. Differences in the Biological Functions of BDNF and proBDNF in the Central Nervous System. Neurosci Behav Physiol (2017) 47(3):251–65. 10.1007/s11055-017-0391-5 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

