Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects
- PMID: 33324405
- PMCID: PMC7723893
- DOI: 10.3389/fimmu.2020.585880
Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects
Abstract
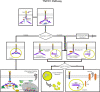
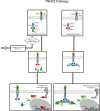
Since its discovery in 1975, TNFα has been a subject of intense study as it plays significant roles in both immunity and cancer. Such attention is well deserved as TNFα is unique in its engagement of pleiotropic signaling via its two receptors: TNFR1 and TNFR2. Extensive research has yielded mechanistic insights into how a single cytokine can provoke a disparate range of cellular responses, from proliferation and survival to apoptosis and necrosis. Understanding the intracellular signaling pathways induced by this single cytokine via its two receptors is key to further revelation of its exact functions in the many disease states and immune responses in which it plays a role. In this review, we describe the signaling complexes formed by TNFR1 and TNFR2 that lead to each potential cellular response, namely, canonical and non-canonical NF-κB activation, apoptosis and necrosis. This is followed by a discussion of data from in vivo mouse and human studies to examine the differential impacts of TNFR1 versus TNFR2 signaling.
Keywords: NF-kappa B; TNF; TNF blockade; TNF receptor; epithelial to mesenchymal transition; signaling/signaling pathways.
Copyright © 2020 Gough and Myles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

