Chimeric Antigen Receptor Based Therapy as a Potential Approach in Autoimmune Diseases: How Close Are We to the Treatment?
- PMID: 33324420
- PMCID: PMC7727445
- DOI: 10.3389/fimmu.2020.603237
Chimeric Antigen Receptor Based Therapy as a Potential Approach in Autoimmune Diseases: How Close Are We to the Treatment?
Abstract
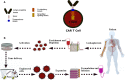
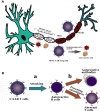
Despite significant breakthroughs in understanding of immunological and physiological features of autoimmune diseases, there is currently no specific therapeutic option with prolonged remission. Cell-based therapy using engineered-T cells has attracted tremendous attention as a practical treatment for autoimmune diseases. Genetically modified-T cells armed with chimeric antigen receptors (CARs) attack autoreactive immune cells such as B cells or antibody-secreting plasma cells. CARs can further guide the effector and regulatory T cells (Tregs) to the autoimmune milieu to traffic, proliferate, and exert suppressive functions. The genetically modified-T cells with artificial receptors are a promising option to suppress autoimmune manifestation and autoinflammatory events. Interestingly, CAR-T cells are modified to a new chimeric auto-antibody receptor T (CAAR-T) cell. This cell, with its specific-antigen, recognizes and binds to the target autoantibodies expressing autoreactive cells and, subsequently, destroy them. Preclinical studies of CAR-T cells demonstrated satisfactory outcomes against autoimmune diseases. However, the lack of target autoantigens remains one of the pivotal problems in the field of CAR-T cells. CAR-based therapy has to pass several hurdles, including stability, durability, trafficking, safety, effectiveness, manufacturing, and persistence, to enter clinical use. The primary goal of this review was to shed light on CAR-T immunotherapy, CAAR-T cell therapy, and CAR-Treg cell therapy in patients with immune system diseases.
Keywords: CAAR-Tregs; CAR-T cells; Tregs; adoptive cell therapy; autoimmune diseases; cytotoxic T cells; immunotherapy.
Copyright © 2020 Sadeqi Nezhad, Seifalian, Bagheri, Yaghoubi, Karimi and Adbollahpour-Alitappeh.
Conflict of interest statement
AS was employed by The London BioScience Innovation Centre. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

