The Role of Paracrine Regulation of Mesenchymal Stem Cells in the Crosstalk With Macrophages in Musculoskeletal Diseases: A Systematic Review
- PMID: 33324622
- PMCID: PMC7726268
- DOI: 10.3389/fbioe.2020.587052
The Role of Paracrine Regulation of Mesenchymal Stem Cells in the Crosstalk With Macrophages in Musculoskeletal Diseases: A Systematic Review
Abstract
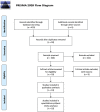
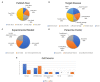
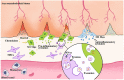
The phenotypic change of macrophages (Mφs) plays a crucial role in the musculoskeletal homeostasis and repair process. Although mesenchymal stem cells (MSCs) have been shown as a novel approach in tissue regeneration, the therapeutic potential of MSCs mediated by the interaction between MSC-derived paracrine mediators and Mφs remains elusive. This review focused on the elucidation of paracrine crosstalk between MSCs and Mφs during musculoskeletal diseases and injury. The search method was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and Cochrane Guidelines. The search strategies included MeSH terms and other related terms of MSC-derived mediators and Mφs. Ten studies formed the basis of this review. The current finding suggested that MSC administration promoted proliferation and activation of CD163+ or CD206+ M2 Mφs in parallel with reduction of proinflammatory cytokines and increase in anti-inflammatory cytokines. During such period, Mφs also induced MSCs into a motile and active phenotype via the influence of proinflammatory cytokines. Such crosstalk between Mφs and MSCs further strengthens the effect of paracrine mediators from MSCs to regulate Mφs phenotypic alteration. In conclusion, MSCs in musculoskeletal system, mediated by the interaction between MSC paracrine and Mφs, have therapeutic potential in musculoskeletal diseases.
Keywords: exosomes; extracellular vesicles (EVs); macrophages; mesenchymal stem cells (MeSH ID D059630); musculoskeletal.
Copyright © 2020 Xu, Lee, Wang, Huang, Shin, Wang, Wan, Zhu, Yung and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aktas E., Chamberlain C. S., Saether E. E., Duenwald-Kuehl S. E., Kondratko-Mittnacht J., Stitgen M., et al. . (2017). Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an achilles tendon segmental defect. J. Orthop. Res. 35, 269–280. 10.1002/jor.23258 - DOI - PubMed
-
- Anderson C. F., Mosser D. M. (2002). A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 72, 101–106. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

