Para-Substituted α-Phenyl- N- tert-butyl Nitrones: Spin-Trapping, Redox and Neuroprotective Properties
- PMID: 33324807
- PMCID: PMC7726753
- DOI: 10.1021/acsomega.0c03907
Para-Substituted α-Phenyl- N- tert-butyl Nitrones: Spin-Trapping, Redox and Neuroprotective Properties
Abstract
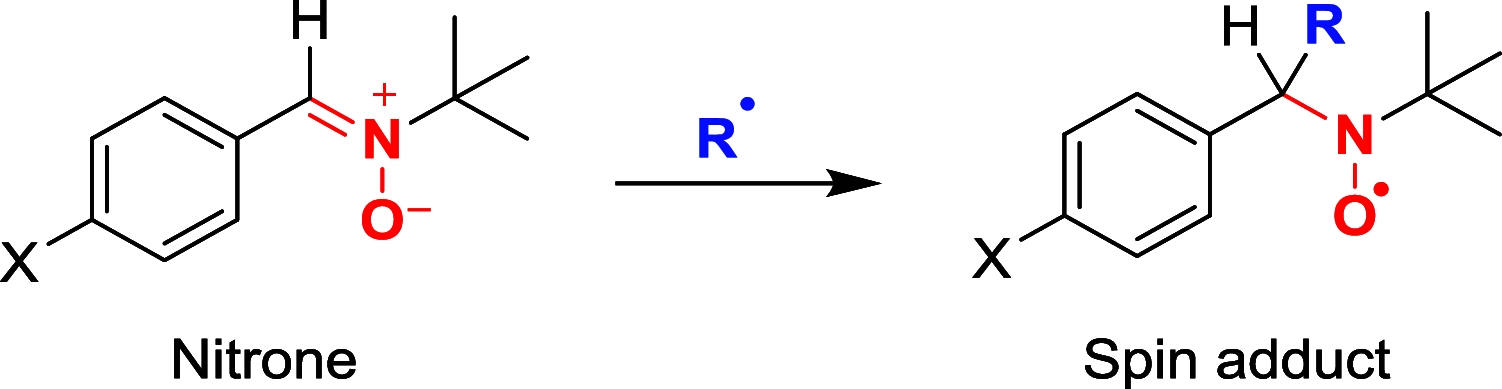
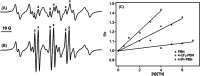
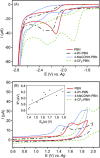
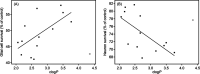
In this work, a series of para-substituted α-phenyl-N-tert-butyl nitrones (PBN) were studied. Their radical-trapping properties were evaluated by electron paramagnetic resonance, with 4-CF3-PBN being the fastest derivative to trap the hydroxymethyl radical (•CH2OH). The redox properties of the nitrones were further investigated by cyclic voltammetry, and 4-CF3-PBN was the easiest to reduce and the hardest to oxidize. This is due to the presence of the electron-withdrawing CF3 group. Very good correlations between the Hammett constants (σp) of the substituents and both spin-trapping rates and redox potentials were observed. These correlations were further supported by computationally determined ionization potentials and atom charge densities. Finally, the neuroprotective effect of these derivatives was studied using two different in vitro models of cell death on primary cortical neurons injured by glutamate exposure or on glial cells exposed to t BuOOH. Trends between the protection afforded by the nitrones and their lipophilicity were observed. 4-CF3-PBN was the most potent agent against t BuOOH-induced oxidative stress on glial cells, while 4-Me2N-PBN showed potency in both models.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Halliwell B.; Gutteridge J. M. C.. Free Radicals in Biology and Medicine; Oxford University Press, 2015.
-
- Villamena F. A.Reactive Species Detection in Biology: From Fluorescence to Electron Paramagnetic Resonance Spectroscopy; Elsevier, 2017.
LinkOut - more resources
Full Text Sources
Other Literature Sources

