Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: Addition to the S1 guideline: Therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society
- PMID: 33324917
- PMCID: PMC7650107
- DOI: 10.1186/s42466-020-00057-1
Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: Addition to the S1 guideline: Therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society
Abstract
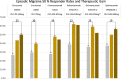
Monoclonal antibodies against the calcitonin gene-related peptide (CGRP) receptor (Erenumab) or against CGRP (Eptinezumab, Fremanezumab, Galcanezumab) are new substances for the preventive treatment of migraine. They represent an extension of the therapeutic options, which already exist in migraine prevention. In randomized, placebo-controlled studies, the efficacy and good tolerability of these specific substances have been demonstrated in patients with episodic and chronic migraine. The following treatment recommendation presents a summary of the pivotal studies. Recommendations are provided for the targeted selection of patients as well as for the evaluation of therapeutic success and the duration of treatment. Finally, possible restrictions on the use of this new substance group are discussed. This guideline is an abridged and translated version of the guideline published by Diener H-C, May A et al., Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor, Supplement to S1 Guideline Therapy of Migraine Attack and Prevention of Migraine, 2019, Deutsche Gesellschaft für Neurologie (eds.), Guidelines for Diagnostics and Therapy in Neurology. A complete version of this guideline can be found on the website of the Deutsche Gesellschaft für Neurologie (www.dgn.org/leitlinien) and the AWMF (Arbeitsgemeinschaft wissenschaftlicher Medizinischer Gesellschaften). This guideline has been approved by the German Neurological Society (DGN) and the German Migraine and Headache Society (GMHS) and was reviewed by the two societies.
Keywords: CGRP; Chronic migraine; Episodic migraine; Guideline; Migraine prevention; Monoclonal antibodies.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsDeclaration of interests and handling of conflicts of interest. All participants in the guideline have submitted their declarations of interest (AWMF form for the declaration of interests in the context of guideline projects) to the coordinator or the Editorial Office Guidelines of the DGN in due time and completely filled out. In the form, those completing the form were asked to indicate whether the interests presented were thematically related to the guideline/guideline topic. If the information provided was incomplete, corrections were requested. They were also asked to indicate the amount of remuneration, which, however, is not published. A self-assessment no longer took place. All declarations of interest were reviewed by an anonymous, independent and knowledgeable conflict of interest officer of the DGN for potential relevant thematic interests. The information was examined with regard to an existing thematic reference, thematic relevance, type and intensity of the relationship and the absolute amount of the remuneration. The following evaluation criteria were applied: • Paid assessor/consultant work for industrial companies • Participation in a scientific advisory board: paid work for industrial companies • Lectures: paid for by the industry • Authorship or co-authorship: only if industry-driven • Research projects/conducting clinical studies: directly or partially financed by industrial companies • Ownership interests (patents, shareholdings) with reference to guidelines • indirect interests with relevance The 50% rule of the DGN is a special requirement of the DGN since May 2014 which stipulates that for a balanced composition of the guideline group, at least 50% of the participants in the guideline must have no or only minor conflicts of interest relevant to the guideline. The DGN has decided to introduce the 50% rule because it prevents any overlap of individual interests in voting. Evaluation of the interests presented The editorial committee consists of 14 members, including two lead authors. Of the total group, 9 members are free of conflicts of interest or have only minor thematically relevant conflicts of interest. 5 contributors with moderate conflicts of interest were not involved in the formulation of the guidelines text. They had an advisory and/or corrective function. The 50% rule of the DGN was observed. For reasons of transparency, the interests of the parties involved and their assessment by DGN conflict of interest officers are listed a summary table on the webpage of the guideline.
Figures


References
-
- Diener H, May A, et al. Prophylaxe der Migräne mit monoklonlane Antikörpern gegen CGRP oder den CGRP Rezeptor. Ergänzung der S1-Leitlinie Therapie der Migräneattacke und Prophylaxe der Migräne. Berlin: Deutsche Gesellschaft für Neurologie; 2019.
-
- Tassorelli, C., Diener, H. C., Dodick, D. W., Silberstein, S. D., Lipton, R. B., Ashina, M., et al. (2018). Guidelines of the international headache society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia, 38(5), 815–832 - PubMed
-
- Goadsby PJ, Edvinsson L. Sumatriptan reverses the changes in calcitonin gene-related peptide seen in the headache phase of migraine. Cephalalgia. 1991;11(Suppl 11):3–4. doi: 10.1177/0333102491011S1102. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
