Tubulin post-translational modifications control neuronal development and functions
- PMID: 33325152
- PMCID: PMC8246997
- DOI: 10.1002/dneu.22774
Tubulin post-translational modifications control neuronal development and functions
Abstract
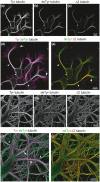
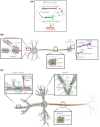
Microtubules (MTs) are an essential component of the neuronal cytoskeleton; they are involved in various aspects of neuron development, maintenance, and functions including polarization, synaptic plasticity, and transport. Neuronal MTs are highly heterogeneous due to the presence of multiple tubulin isotypes and extensive post-translational modifications (PTMs). These PTMs-most notably detyrosination, acetylation, and polyglutamylation-have emerged as important regulators of the neuronal microtubule cytoskeleton. With this review, we summarize what is currently known about the impact of tubulin PTMs on microtubule dynamics, neuronal differentiation, plasticity, and transport as well as on brain function in normal and pathological conditions, in particular during neuro-degeneration. The main therapeutic approaches to neuro-diseases based on the modulation of tubulin PTMs are also summarized. Overall, the review indicates how tubulin PTMs can generate a large number of functionally specialized microtubule sub-networks, each of which is crucial to specific neuronal features.
Keywords: neuro-diseases; neuron; post-translational modifications; tubulin; tyrosination.
© 2020 The Authors. Developmental Neurobiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

