Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis
- PMID: 33326026
- PMCID: PMC7745099
- DOI: 10.1001/jamanetworkopen.2020.30097
Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis
Abstract
Importance: Standard therapy for locally advanced rectal cancer includes concurrent chemoradiotherapy followed by surgery and adjuvant chemotherapy (CRT plus A). An alternative strategy known as total neoadjuvant therapy (TNT) involves administration of CRT plus neoadjuvant chemotherapy before surgery with the goal of delivering uninterrupted systemic therapy to eradicate micrometastases. A comparison of these 2 approaches has not been systematically reviewed previously.
Objective: To determine the differences in rates of pathologic complete response (PCR), disease-free and overall survival, sphincter-preserving surgery, and ileostomy between patients receiving TNT vs standard CRT plus A.
Data sources: MEDLINE (via PubMed) and Embase (via OVID) were searched from inception through July 1, 2020, for the following terms: anal/anorectal neoplasms OR anal/anorectal cancer AND total neoadjuvant treatment OR total neoadjuvant therapy. Only studies in English were included.
Study selection: Randomized clinical trials or prospective/retrospective cohort studies comparing outcomes in patients with locally advanced rectal cancer who received TNT vs CRT plus A.
Data extraction and synthesis: Data regarding the first author, publication year, location, sample size, and rates of PCR, sphincter-preserving surgery, ileostomy, and disease-free and overall survival were extracted using Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and pooled using a random-effects model.
Main outcomes and measures: Rates of PCR, sphincter-preserving surgery, ileostomy, and disease-free and overall survival.
Results: After reviewing 2165 reports, 7 unique studies including a total of 2416 unique patients, of whom 1206 received TNT, were selected. The median age for the patients receiving TNT ranged from 57 to 69 years, with 58% to 73% being male. The pooled prevalence of PCR was 29.9% (range, 17.2%-38.5%) in the TNT group and 14.9% (range, 4.2%-21.3%) in the CRT plus A group. Total neoadjuvant therapy was associated with a higher chance of achieving a PCR (odds ratio [OR], 2.44; 95% CI, 1.99-2.98). No statistically significant difference in the proportion of sphincter-preserving surgery (OR, 1.06; 95% CI, 0.73-1.54) or ileostomy (OR, 1.05; 95% CI, 0.76-1.46) between recipients of TNT and CRT plus A was observed. Only 3 studies presented data on disease-free survival, and pooled analysis showed significantly higher odds of improved disease-free survival in patients who received TNT (OR, 2.07; 95% CI, 1.20-3.56; I2 = 49%). Data on overall survival were not consistently reported.
Conclusions and relevance: The findings of this systematic review and meta-analysis suggest that TNT is a promising strategy in locally advanced rectal cancer, with superior rates of PCR compared with standard therapy. However, the long-term effect on disease recurrence and overall survival needs to be explored in future studies.
Conflict of interest statement
Figures


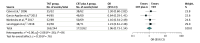
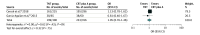

Comment in
-
Personalizing Treatment for Rectal Cancer: Total Neoadjuvant Therapy Is Leading the Way.JAMA Netw Open. 2020 Dec 1;3(12):e2030508. doi: 10.1001/jamanetworkopen.2020.30508. JAMA Netw Open. 2020. PMID: 33326022 No abstract available.
References
-
- Bosset JF, Calais G, Mineur L, et al. ; EORTC Radiation Oncology Group . Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184-190. doi:10.1016/S1470-2045(13)70599-0 - DOI - PubMed
-
- Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. . No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223-229. doi:10.1016/j.radonc.2014.10.006 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

