Isolation and Pharmacological Characterization of Six Opioidergic Picralima nitida Alkaloids
- PMID: 33326237
- PMCID: PMC7932029
- DOI: 10.1021/acs.jnatprod.0c01036
Isolation and Pharmacological Characterization of Six Opioidergic Picralima nitida Alkaloids
Abstract
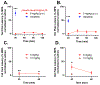
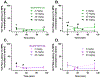
The seeds of the akuamma tree (Picralima nitida) have been used as a traditional treatment for pain and fever. Previous studies have attributed these effects to a series of indole alkaloids found within the seed extracts; however, these pharmacological studies were significantly limited in scope. Herein, an isolation protocol employing pH-zone-refining countercurrent chromatography was developed to provide six of the akuamma alkaloids in high purity and quantities sufficient for more extensive biological evaluation. Five of these alkaloids, akuammine (1), pseudo-akuammigine (3), akuammicine (4), akuammiline (5), and picraline (6), were evaluated against a panel of >40 central nervous system receptors to identify that their primary targets are the opioid receptors. Detailed in vitro investigations revealed 4 to be a potent kappa opioid receptor agonist, and three alkaloids (1-3) were shown to have micromolar activity at the mu opioid receptor. The mu opioid receptor agonists were further evaluated for analgesic properties but demonstrated limited efficacy in assays of thermal nociception. These findings contradict previous reports of the antinociceptive properties of the P. nitida alkaloids and the traditional use of akuamma seeds as analgesics. Nevertheless, their opioid-preferring activity does suggest the akuamma alkaloids provide distinct scaffolds from which novel opioids with unique pharmacologic properties and therapeutic utility can be developed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Hylands-White N; Duarte RV; Raphael JH Rheumatol. Int. 2017, 37, 29–42. - PubMed
-
- Ho A; Nair S In Developments in Neuroethics and Bioethics, Vol. 1; Global Chronic Pain: Public and Population Health Responses; Buchman DZ; Davis KD, Eds. Academic Press: Cambridge, MA, 2018; pp 171–189.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

