The H+-ATPase (V-ATPase): from proton pump to signaling complex in health and disease
- PMID: 33326313
- PMCID: PMC8294626
- DOI: 10.1152/ajpcell.00442.2020
The H+-ATPase (V-ATPase): from proton pump to signaling complex in health and disease
Abstract
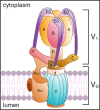
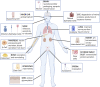
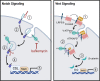
A primary function of the H+-ATPase (or V-ATPase) is to create an electrochemical proton gradient across eukaryotic cell membranes, which energizes fundamental cellular processes. Its activity allows for the acidification of intracellular vesicles and organelles, which is necessary for many essential cell biological events to occur. In addition, many specialized cell types in various organ systems such as the kidney, bone, male reproductive tract, inner ear, olfactory mucosa, and more, use plasma membrane V-ATPases to perform specific activities that depend on extracellular acidification. It is, however, increasingly apparent that V-ATPases are central players in many normal and pathophysiological processes that directly influence human health in many different and sometimes unexpected ways. These include cancer, neurodegenerative diseases, diabetes, and sensory perception, as well as energy and nutrient-sensing functions within cells. This review first covers the well-established role of the V-ATPase as a transmembrane proton pump in the plasma membrane and intracellular vesicles and outlines factors contributing to its physiological regulation in different cell types. This is followed by a discussion of the more recently emerging unconventional roles for the V-ATPase, such as its role as a protein interaction hub involved in cell signaling, and the (patho)physiological implications of these interactions. Finally, the central importance of endosomal acidification and V-ATPase activity on viral infection will be discussed in the context of the current COVID-19 pandemic.
Keywords: acidification; endosomal trafficking; pH regulation; pathophysiology; proton pumping ATPase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

