Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: interim analysis after 8 years of follow-up
- PMID: 33326342
- PMCID: PMC8018381
- DOI: 10.1080/21645515.2020.1839292
Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: interim analysis after 8 years of follow-up
Abstract
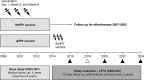
A long-term follow-up (LTFU) of the nine-valent human papillomavirus (9vHPV) vaccine efficacy study in young women aged 16-26 years was initiated to evaluate if vaccine effectiveness for up to 14 years post-vaccination will remain above 90%. Vaccine effectiveness is measured as percent reduction in the incidence of HPV16/18/31/33/45/52/58-related high-grade cervical dysplasia in the LTFU cohort relative to expected incidence in a similar unvaccinated cohort. We report an interim analysis 8 years post-vaccination. Overall, 2029 participants from Denmark, Norway, and Sweden who received the 9vHPV vaccine during the clinical efficacy study continued into the LTFU study. National health registries were used to identify screening attendance and cervical pre-cancer/cancer diagnoses. Tissue samples were retrieved for HPV testing by PCR and pathology diagnosis adjudication. A control chart method was used to detect signals indicative of vaccine effectiveness waning below 90%. No new cases of HPV16/18/31/33/45/52/58-related high-grade cervical dysplasia were observed during the LTFU study period over 4084.2 person-years' follow-up (per-protocol effectiveness population; n = 1448). Thus, there were no signals indicative of vaccine effectiveness waning below 90%. These observations show that the 9vHPV vaccine provides continued statistically significant protection through at least 6 years, with indications of continued effectiveness through 8 years.
Trial registration: Clinicaltrials.gov: NCT00543543, NCT02653118.
Keywords: Cervical intraepithelial neoplasia; effectiveness; human papillomavirus; long-term follow-up; nine-valent human papillomavirus vaccine; vaccine.
Figures

References
-
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–59. - PubMed
-
- Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen O-E, Kjaer SK, Ferenczy A, Kurman RJ, Ronnett BM, Stoler MH, et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019;154(1):110–17. doi: 10.1016/j.ygyno.2019.03.253. - DOI - PubMed
-
- Luxembourg A, Kjaer SK, Nygard M, Ellison MC, Group T, Marshall JB, Radley D, Saah A.. Design of a long-term follow-up effectiveness, immunogenicity and safety study of women who received the 9-valent human papillomavirus vaccine. Contemp Clin Trials. 2017;52:54–61. doi: 10.1016/j.cct.2016.10.006. - DOI - PubMed
