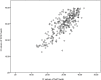
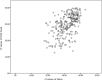
The accuracy of healthcare worker versus self collected (2-in-1) Oropharyngeal and Bilateral Mid-Turbinate (OPMT) swabs and saliva samples for SARS-CoV-2
- PMID: 33326503
- PMCID: PMC7744114
- DOI: 10.1371/journal.pone.0244417
The accuracy of healthcare worker versus self collected (2-in-1) Oropharyngeal and Bilateral Mid-Turbinate (OPMT) swabs and saliva samples for SARS-CoV-2
Abstract
Background: Self-sampling for SARS-CoV-2 would significantly raise testing capacity and reduce healthcare worker (HCW) exposure to infectious droplets personal, and protective equipment (PPE) use.
Methods: We conducted a diagnostic accuracy study where subjects with a confirmed diagnosis of COVID-19 (n = 401) and healthy volunteers (n = 100) were asked to self-swab from their oropharynx and mid-turbinate (OPMT), and self-collect saliva. The results of these samples were compared to an OPMT performed by a HCW in the same patient at the same session.
Results: In subjects confirmed to have COVID-19, the sensitivities of the HCW-swab, self-swab, saliva, and combined self-swab plus saliva samples were 82.8%, 75.1%, 74.3% and 86.5% respectively. All samples obtained from healthy volunteers were tested negative. Compared to HCW-swab, the sensitivities of a self-swab sample and saliva sample were inferior by 8.7% (95%CI: 2.4% to 15.0%, p = 0.006) and 9.5% (95%CI: 3.1% to 15.8%, p = 0.003) respectively. The combined detection rate of self-swab and saliva had a sensitivity of 2.7% (95%CI: -2.6% to 8.0%, p = 0.321). The sensitivity of both the self-collection methods are higher when the Ct value of the HCW swab is less than 30. The specificity of both the self-swab and saliva testing was 100% (95% CI 96.4% to 100%).
Conclusion: Our study provides evidence that sensitivities of self-collected OPMT swab and saliva samples were inferior to a HCW swab, but they could still be useful testing tools in the appropriate clinical settings.
Conflict of interest statement
Author CXT is employed by Temasek International Pte Ltd, and was acting on behalf of Sheares Healthcare Group Pte Ltd for the study. This commercial affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Comparison of saliva with healthcare workers- and patient-collected swabs in the diagnosis of COVID-19 in a large cohort.BMC Infect Dis. 2021 Jul 5;21(1):648. doi: 10.1186/s12879-021-06343-w. BMC Infect Dis. 2021. PMID: 34225656 Free PMC article.
-
Validation of self-collected buccal swab and saliva as a diagnostic tool for COVID-19.Int J Infect Dis. 2021 Mar;104:255-261. doi: 10.1016/j.ijid.2020.12.080. Epub 2021 Jan 2. Int J Infect Dis. 2021. PMID: 33401035 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs : A Systematic Review and Meta-analysis.Ann Intern Med. 2021 Apr;174(4):501-510. doi: 10.7326/M20-6569. Epub 2021 Jan 12. Ann Intern Med. 2021. PMID: 33428446 Free PMC article.
-
Screening for SARS-CoV-2 by RT-PCR: Saliva or nasopharyngeal swab? Rapid review and meta-analysis.PLoS One. 2021 Jun 10;16(6):e0253007. doi: 10.1371/journal.pone.0253007. eCollection 2021. PLoS One. 2021. PMID: 34111196 Free PMC article.
Cited by
-
Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: a Diagnostic Accuracy Study.Microbiol Spectr. 2022 Feb 23;10(1):e0202921. doi: 10.1128/spectrum.02029-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107327 Free PMC article.
-
Implementation and User Satisfaction of a Comprehensive Telemedicine Approach for SARS-CoV-2 Self-Sampling: Monocentric, Prospective, Interventional, Open-Label, Controlled, Two-Arm Feasibility Study.JMIR Form Res. 2024 Dec 11;8:e57608. doi: 10.2196/57608. JMIR Form Res. 2024. PMID: 39661941 Free PMC article. Clinical Trial.
-
Self-sampling versus health care professional-guided swab collection for SARS-CoV-2 testing.Infection. 2021 Oct;49(5):927-934. doi: 10.1007/s15010-021-01614-9. Epub 2021 May 10. Infection. 2021. PMID: 33970430 Free PMC article.
-
Diagnostic performance of oral swab specimen for SARS-CoV-2 detection with rapid point-of-care lateral flow antigen test.Sci Rep. 2022 May 5;12(1):7355. doi: 10.1038/s41598-022-11284-8. Sci Rep. 2022. PMID: 35513547 Free PMC article.
-
A Telemedicine-Guided Self-Collection Approach for PCR-Based SARS-CoV-2 Testing: Comparative Study.JMIR Form Res. 2022 Jan 4;6(1):e32564. doi: 10.2196/32564. JMIR Form Res. 2022. PMID: 34803022 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous