Leveraging phenotypic variability to identify genetic interactions in human phenotypes
- PMID: 33326753
- PMCID: PMC7820920
- DOI: 10.1016/j.ajhg.2020.11.016
Leveraging phenotypic variability to identify genetic interactions in human phenotypes
Abstract
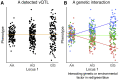
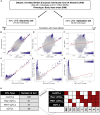
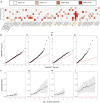
Although thousands of loci have been associated with human phenotypes, the role of gene-environment (GxE) interactions in determining individual risk of human diseases remains unclear. This is partly because of the severe erosion of statistical power resulting from the massive number of statistical tests required to detect such interactions. Here, we focus on improving the power of GxE tests by developing a statistical framework for assessing quantitative trait loci (QTLs) associated with the trait means and/or trait variances. When applying this framework to body mass index (BMI), we find that GxE discovery and replication rates are significantly higher when prioritizing genetic variants associated with the variance of the phenotype (vQTLs) compared to when assessing all genetic variants. Moreover, we find that vQTLs are enriched for associations with other non-BMI phenotypes having strong environmental influences, such as diabetes or ulcerative colitis. We show that GxE effects first identified in quantitative traits such as BMI can be used for GxE discovery in disease phenotypes such as diabetes. A clear conclusion is that strong GxE interactions mediate the genetic contribution to body weight and diabetes risk.
Keywords: GWAS; GxE; body mass index; complex traits; diabetes; gene-environment interactions; phenotypic variance; vQTL.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
O.E. is scientific advisor and equity holder in Freenome, Owkin, Volastra Therapeutics, and One Three Biotech. C.V.V.H. is an employee of the Regeneron Genetics Center.
Figures


References
-
- Carbone M., Emri S., Dogan A.U., Steele I., Tuncer M., Pass H.I., Baris Y.I. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat. Rev. Cancer. 2007;7:147–154. - PubMed
-
- Roushdy-Hammady I., Siegel J., Emri S., Testa J.R., Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357:444–445. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

