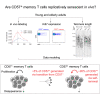
CD57+ Memory T Cells Proliferate In Vivo
- PMID: 33326780
- PMCID: PMC7758161
- DOI: 10.1016/j.celrep.2020.108501
CD57+ Memory T Cells Proliferate In Vivo
Abstract
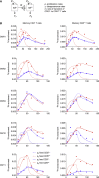
A central paradigm in the field of lymphocyte biology asserts that replicatively senescent memory T cells express the carbohydrate epitope CD57. These cells nonetheless accumulate with age and expand numerically in response to persistent antigenic stimulation. Here, we use in vivo deuterium labeling and ex vivo analyses of telomere length, telomerase activity, and intracellular expression of the cell-cycle marker Ki67 to distinguish between two non-exclusive scenarios: (1) CD57+ memory T cells do not proliferate and instead arise via phenotypic transition from the CD57- memory T cell pool; and/or (2) CD57+ memory T cells self-renew via intracompartmental proliferation. Our results provide compelling evidence in favor of the latter scenario and further suggest in conjunction with mathematical modeling that self-renewal is by far the most abundant source of newly generated CD57+ memory T cells. Immunological memory therefore appears to be intrinsically sustainable among highly differentiated subsets of T cells that express CD57.
Keywords: Enter keywords here.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
-
- Abo T., Balch C.M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J. Immunol. 1981;127:1024–1029. - PubMed
-
- Addo M.M., Draenert R., Rathod A., Verrill C.L., Davis B.T., Gandhi R.T., Robbins G.K., Basgoz N.O., Stone D.R., Cohen D.E. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS ONE. 2007;2:e321. - PMC - PubMed
-
- Appay V., van Lier R.A., Sallusto F., Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 29202/CRUK_/Cancer Research UK/United Kingdom
- R01 AI043866/AI/NIAID NIH HHS/United States
- 100326/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- 093053/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- 103865/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- MR/P001602/1/MRC_/Medical Research Council/United Kingdom
- R37 AI040312/AI/NIAID NIH HHS/United States
- 18246/CRUK_/Cancer Research UK/United Kingdom
- UL1 RR024131/RR/NCRR NIH HHS/United States
- DP1 OD000329/OD/NIH HHS/United States
- C17199/A18246/CRUK_/Cancer Research UK/United Kingdom
- MR/J007439/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G1001052/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

