Asynchronous development of Zymoseptoria tritici infection in wheat
- PMID: 33326850
- PMCID: PMC7812371
- DOI: 10.1016/j.fgb.2020.103504
Asynchronous development of Zymoseptoria tritici infection in wheat
Abstract
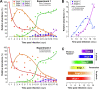
The fungus Zymoseptoria tritici causes Septoria tritici blotch of wheat. Pathogenicity begins with spore germination, followed by stomata invasion by hyphae, mesophyll colonization and fruiting body formation. It was previously found that entry into the plant via stomata occurs in a non-synchronized way over several days, while later developmental steps, such as early and late fruiting body formation, were reported to follow each other in time. This suggests synchronization of the pathogen population in planta prior to sporulation. Here, we image a fluorescent Z. tritici IPO323-derived strain during infection. We describe 6 morphologically distinct developmental stages, and determine their abundance in infected leaves, with time post inoculation. This demonstrates that 3-5 stages co-exist in infected tissues at any given time. Thus, later stages of pathogen development also occur asynchronously amongst the population of infecting cells. This merits consideration when interpreting transcriptomics or proteomics data gathered from infected plants.
Copyright © 2020. Published by Elsevier Inc.
Figures


References
-
- Brennan C.J., Benbowa H.R., Mullinsband E., Doohan F.M. A review of the known unknowns in the early stages of septoria tritici blotch disease of wheat. Plant Pathol. 2019;68:1427–1438.
-
- Deller S., Hammond-Kosack K.E., Rudd J.J. The complex interactions between host immunity and non-biotrophic fungal pathogens of wheat leaves. J. Plant Phys. 2011;168:63–71. - PubMed
-
- Duncan K.E., Howard R.J. Cytological analysis of wheat infection by the leaf blotch pathogen Mycosphaerella graminicola. Mycol Res. 2000;104:1074–1082.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

