Water and lipid suppression techniques for advanced 1 H MRS and MRSI of the human brain: Experts' consensus recommendations
- PMID: 33327042
- PMCID: PMC8569948
- DOI: 10.1002/nbm.4459
Water and lipid suppression techniques for advanced 1 H MRS and MRSI of the human brain: Experts' consensus recommendations
Abstract
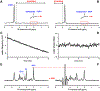
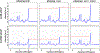
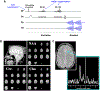
The neurochemical information provided by proton magnetic resonance spectroscopy (MRS) or MR spectroscopic imaging (MRSI) can be severely compromised if strong signals originating from brain water and extracranial lipids are not properly suppressed. The authors of this paper present an overview of advanced water/lipid-suppression techniques and describe their advantages and disadvantages. Moreover, they provide recommendations for choosing the most appropriate techniques for proper use. Methods of water signal handling are primarily focused on the VAPOR technique and on MRS without water suppression (metabolite cycling). The section on lipid-suppression methods in MRSI is divided into three parts. First, lipid-suppression techniques that can be implemented on most clinical MR scanners (volume preselection, outer-volume suppression, selective lipid suppression) are described. Second, lipid-suppression techniques utilizing the combination of k-space filtering, high spatial resolutions and lipid regularization are presented. Finally, three promising new lipid-suppression techniques, which require special hardware (a multi-channel transmit system for dynamic B1+ shimming, a dedicated second-order gradient system or an outer volume crusher coil) are introduced.
Keywords: ECLIPSE; L-regularization; VAPOR; crusher coils; dynamic B1 shimming; high spatial resolution; inversion recovery; metabolite cycling.
© 2020 John Wiley & Sons, Ltd.
Figures


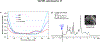



References
-
- McIntyre DJ, Charlton RA, Markus HS, Howe FA. Long and short echo time proton magnetic resonance spectroscopic imaging of the healthy aging brain. J Magn Reson Imaging. 2007;26(6):1596–1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

