Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer
- PMID: 33327047
- PMCID: PMC7930437
- DOI: 10.3802/jgo.2021.32.e16
Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer
Abstract
Objective: To evaluate the efficacy and safety of niraparib in Japanese women with heavily pretreated ovarian cancer.
Methods: This Phase 2 open-label, single-arm study enrolled Japanese women with homologous recombination deficiency-positive relapsed, high-grade serous ovarian, fallopian tube, or primary peritoneal cancer who had completed 3-4 lines of therapy. The starting dose of niraparib was 300 mg administered once daily in continuous 28-day cycles until objective progressive disease, unacceptable toxicity, consent withdrawal or discontinuation. The primary endpoint, objective response rate (ORR), was assessed by the investigator using RECIST version 1.1. Safety evaluations included the incidence of treatment-emergent adverse events (TEAEs), including serious TEAEs.
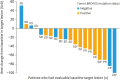
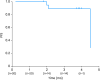
Results: Twenty women were enrolled and the confirmed ORR in the full analysis set (FAS) was 35.0% (7/20), consisting of 1 complete response and 6 partial responses. Disease control rate in the FAS was 90.0%. The most frequently reported TEAEs (>50%) were anemia, nausea, and platelet count decreased. One patient (5.0%) had TEAEs leading to discontinuation of niraparib whereas reductions or interruptions were reported in 14 (70.0%) and 15 (75.0%) patients, respectively. The median dose intensity (202.9 mg daily) corresponded to a relative dose intensity of 67.6%.
Conclusion: Efficacy and safety of niraparib in heavily pretreated Japanese women was comparable to that seen in an equivalent population of non-Japanese women. No new safety signals were identified.
Trial registration: ClinicalTrials.gov Identifier: NCT03759600.
Keywords: Japanese; Late-line Treatment; Niraparib; Ovarian Cancer; Phase 2; Salvage.
Copyright © 2021. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Figures
Comment in
-
How to start niraparib in real-world Asian ovarian cancer patients?J Gynecol Oncol. 2021 Mar;32(2):e36. doi: 10.3802/jgo.2021.32.e36. Epub 2021 Feb 8. J Gynecol Oncol. 2021. PMID: 33559417 Free PMC article. No abstract available.
References
-
- Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP inhibition in cancer: an update on clinical development. Target Oncol. 2019;14:657–679. - PubMed
-
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. - PubMed
-
- González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. - PubMed
-
- Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

