Albanol B from Mulberries Exerts Anti-Cancer Effect through Mitochondria ROS Production in Lung Cancer Cells and Suppresses In Vivo Tumor Growth
- PMID: 33327489
- PMCID: PMC7764986
- DOI: 10.3390/ijms21249502
Albanol B from Mulberries Exerts Anti-Cancer Effect through Mitochondria ROS Production in Lung Cancer Cells and Suppresses In Vivo Tumor Growth
Abstract
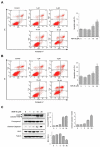
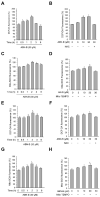
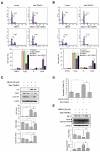
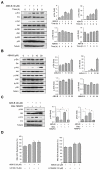
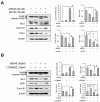
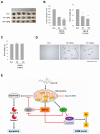
Albanol B (ABN-B), an arylbenzofuran derivative isolated from mulberries, has been shown to have anti-Alzheimer's disease, anti-bacterial and antioxidant activities. The aim of this study was to investigate the anti-cancer effect of this compound against lung cancer cells. The results show that ABN-B inhibited the proliferation of four human lung cancer cell lines (A549, BZR, H1975, and H226) and induced apoptosis, based on the cleavage of caspase-7 and PARP (poly (ADP-ribose) polymerase), as well as the downregulation of Bcl-2. ABN-B also induced cell cycle arrest at G2/M by down-regulating the expression of CKD1 (cyclin-dependent kinase 1) and cyclin B1, but up-regulating p21 (cyclin-dependent kinase inhibitor 1) expression. Notably, ABN-B increased the production of mitochondrial reactive oxygen species (ROS); however, treatment with mito-TEMPO (a specific mitochondrial antioxidant) blocked ABN-B-induced cell cycle arrest at G2/M and apoptosis, as well as the up-regulation of p21 and down-regulation of CDK1 and cyclin B1 induced by ABN-B. At the molecular level, ABN-B-induced mitochondrial ROS production increased the phosphorylation levels of AKT (protein kinase B) and ERK1/2 (extracellular signal-regulated kinase 1/2), while the inhibition of these kinases blocked the ABN-B-induced up-regulation of p21 and down-regulation of CDK1 and cyclin B1. Moreover, ABN-B significantly suppressed tumor growth in Ex-3LL (Lewis lung carcinoma) tumor-bearing mice. Taken together, these results suggest that ABN-B can exert an anti-cancer effect by inducing apoptosis and cell cycle arrest at G2/M through mitochondrial ROS production in lung cancer cells.
Keywords: Albanol B; apoptosis; cell cycle arrest; lung cancer; mitochondrial reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

