A Novel Liquid Biopsy Strategy to Detect Small Amounts of Cancer Cells Using Cancer-Specific Replication Adenoviruses
- PMID: 33327605
- PMCID: PMC7765046
- DOI: 10.3390/jcm9124044
A Novel Liquid Biopsy Strategy to Detect Small Amounts of Cancer Cells Using Cancer-Specific Replication Adenoviruses
Abstract
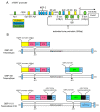
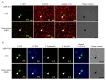
Circulating tumor cells (CTCs) are a promising source of clinical and biological cancer information and can be a material for liquid biopsy. However, detecting and capturing these cells remains a challenge. Various biological factors (e.g., cell surface proteins, cell size, deformability, or dielectrophoresis) have been applied to detect CTCs. Cancer cells dramatically change their characteristics during tumorigenesis and metastasis. Hence, defining a cell as malignant using such a parameter is difficult. Moreover, immortality is an essential characteristic of cancer cells. Telomerase elongates telomeres and plays a critical role in cellular immortality and is specifically activated in cancer cells. Thus, the activation of telomerase can be a good fingerprint for cancer cells. Telomerase cannot be recognized by antibodies in living cells because it is a nuclear enzyme. Therefore, telomerase-specific replication adenovirus, which expresses the green fluorescent protein, has been applied to detect CTCs. This review explores the overview of this novel technology and its application in gynecological cancers.
Keywords: adenovirus; circulating tumor cells (CTCs); telomerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G.J., Uhr J.W., Terstappen L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. - DOI - PubMed
-
- Zhu P., Stanton M.L., Castle E.P., Joseph R.W., Adams D.L., Li S., Amstutz P., Tang C.-M., Ho T.H. Detection of tumor-associated cells in cryopreserved peripheral blood mononuclear cell samples for retrospective analysis. J. Transl. Med. 2016;14:198. doi: 10.1186/s12967-016-0953-2. - DOI - PMC - PubMed
-
- Adams D.L., Adams D.K., Alpaugh R.K., Cristofanilli M., Martin S.S., Chumsri S., Tang C.-M., Marks J.R. Circulating Cancer-Associated Macrophage-Like Cells Differentiate Malignant Breast Cancer and Benign Breast Conditions. Cancer Epidemiol. Biomarkers Prev. 2016;25:1037–1042. doi: 10.1158/1055-9965.EPI-15-1221. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

