A Comprehensive Review of the Immunological Response against Foot-and-Mouth Disease Virus Infection and Its Evasion Mechanisms
- PMID: 33327628
- PMCID: PMC7765147
- DOI: 10.3390/vaccines8040764
A Comprehensive Review of the Immunological Response against Foot-and-Mouth Disease Virus Infection and Its Evasion Mechanisms
Abstract
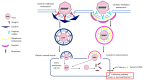
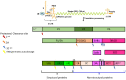
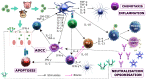
Foot-and-mouth disease (FMD) is a highly contagious viral disease, which has been reported for over 100 years, and against which the struggle has lasted for the same amount of time. It affects individuals from the order Artiodactyla, such as cattle, swine, sheep, wild animals from this order, and a few non-cloven hoofed species, such as mice and elephants. FMD causes large-scale economic losses for agricultural production systems; morbidity is almost 100% in an affected population, accompanied by a high mortality rate in young animals due to myocarditis or an inability to suckle if a mother is ill. The aetiological agent is an Aphthovirus from the family Picornaviridae, having seven serotypes: A, O, C, SAT1, SAT2, SAT3, and Asia 1. Serotype variability means that an immune response is serospecific and vaccines are thus designed to protect against each serotype independently. A host's adaptive immune response is key in defence against pathogens; however, this virus uses successful strategies (along with most microorganisms) enabling it to evade a host's immune system to rapidly and efficiently establish itself within such host, and thus remain there. This review has been aimed at an in-depth analysis of the immune response in cattle and swine regarding FMD virus, the possible evasion mechanisms used by the virus and describing some immunological differences regarding these species. Such aspects can provide pertinent knowledge for developing new FMD control and prevention strategies.
Keywords: foot-and-mouth-disease virus; immune evasion mechanism; immune response.
Conflict of interest statement
The authors declare that there are no conflict of interest regarding the publication of this paper.
Figures

References
-
- Morante Yamir L., Guibarra Escobar V.H. Barreras anatómicas del sistema inmunitario. Rev. Actual. Clín. 2011;13:634–638.
-
- Campos-Granados C. El sistema inmune en los mamíferos: Las defensas del cuerpo. Nutr. Anim. Trop. 2014;8:80–93.
-
- Doménech A., Gibello A., Collado V.M., Porras R., Blanco M. The innate immune system II: First response against infection. Rev. Complut. Cienc. Vet. 2008;2:17–30.
-
- Klossek J.M., Fontanel D.J.P. Fisiología de la mucosa respiratoria nasal y trastornos funcionales. EMC Otorrinolaringol. 2000;38:1–11. doi: 10.1016/s1632-3475(09)70290-5. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

