Efficacy and Safety of Adjunctive Cilostazol to Clopidogrel-Treated Diabetic Patients With Symptomatic Lower Extremity Artery Disease in the Prevention of Ischemic Vascular Events
- PMID: 33327737
- PMCID: PMC7955466
- DOI: 10.1161/JAHA.120.018184
Efficacy and Safety of Adjunctive Cilostazol to Clopidogrel-Treated Diabetic Patients With Symptomatic Lower Extremity Artery Disease in the Prevention of Ischemic Vascular Events
Abstract
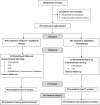
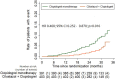
Background Type 2 diabetes mellitus is a risk factor for lower extremity arterial disease. Cilostazol expresses antiplatelet, anti-inflammatory, and vasodilator actions and improves the claudication intermittent symptoms. We investigated the efficacy and safety of adjunctive cilostazol to clopidogrel-treated patients with type 2 diabetes mellitus exhibiting symptomatic lower extremity arterial disease, in the prevention of ischemic vascular events and improvement of the claudication intermittent symptoms. Methods and Results In a prospective 2-arm, multicenter, open-label, phase 4 trial, patients with type 2 diabetes mellitus with intermittent claudication receiving clopidogrel (75 mg/d) for at least 6 months, were randomly assigned in a 1:1 ratio, either to continue to clopidogrel monotherapy, without receiving placebo cilostazol (391 patients), or to additionally receive cilostazol, 100 mg twice/day (403 patients). The median duration of follow-up was 27 months. The primary efficacy end point, the composite of acute ischemic stroke/transient ischemic attack, acute myocardial infarction, and death from vascular causes, was significantly reduced in patients receiving adjunctive cilostazol compared with the clopidogrel monotherapy group (sex-adjusted hazard ratio [HR], 0.468; 95% CI, 0.252-0.870; P=0.016). Adjunctive cilostazol also significantly reduced the stroke/transient ischemic attack events (sex-adjusted HR, 0.38; 95% CI, 0.15-0.98; P=0.046) and improved the ankle-brachial index and pain-free walking distance values (P=0.001 for both comparisons). No significant difference in the bleeding events, as defined by Bleeding Academic Research Consortium criteria, was found between the 2 groups (sex-adjusted HR, 1.080; 95% CI, 0.579-2.015; P=0.809). Conclusions Adjunctive cilostazol to clopidogrel-treated patients with type 2 diabetes mellitus with symptomatic lower extremity arterial disease may lower the risk of ischemic events and improve intermittent claudication symptoms, without increasing the bleeding risk, compared with clopidogrel monotherapy. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT02983214.
Keywords: cilostazol; clopidogrel; coronary artery disease; diabetes mellitus; intermittent claudication; ischemic stroke.
Conflict of interest statement
None.
Figures

References
-
- Firnhaber JM, Powell CS. Lower extremity peripheral artery disease: diagnosis and treatment. Am Fam Physician. 2019;99:362–369. - PubMed
-
- Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al; ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: endorsed by the European Stroke Organization (ESO): the Task Force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. - PubMed
-
- Hossain P, Kokkinidis DG, Armstrong EJ. How to assess a claudication and when to intervene. Curr Cardiol Rep. 2019;21:138. - PubMed
-
- Meru AV, Mittra S, Thyagarajan B, Chugh A. Intermittent claudication: an overview. Atherosclerosis. 2006;187:221–237. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

