Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis
- PMID: 33328245
- PMCID: PMC9231288
- DOI: 10.1136/gutjnl-2020-322771
Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis
Abstract
Objective: Necrotising enterocolitis (NEC) is a devastating intestinal disease primarily affecting preterm infants. The underlying mechanisms are poorly understood: mother's own breast milk (MOM) is protective, possibly relating to human milk oligosaccharide (HMO) and infant gut microbiome interplay. We investigated the interaction between HMO profiles and infant gut microbiome development and its association with NEC.
Design: We performed HMO profiling of MOM in a large cohort of infants with NEC (n=33) with matched controls (n=37). In a subset of 48 infants (14 with NEC), we also performed longitudinal metagenomic sequencing of infant stool (n=644).
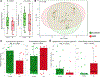
Results: Concentration of a single HMO, disialyllacto-N-tetraose (DSLNT), was significantly lower in MOM received by infants with NEC compared with controls. A MOM threshold level of 241 nmol/mL had a sensitivity and specificity of 0.9 for NEC. Metagenomic sequencing before NEC onset showed significantly lower relative abundance of Bifidobacterium longum and higher relative abundance of Enterobacter cloacae in infants with NEC. Longitudinal development of the microbiome was also impacted by low MOM DSLNT associated with reduced transition into preterm gut community types dominated by Bifidobacterium spp and typically observed in older infants. Random forest analysis combining HMO and metagenome data before disease accurately classified 87.5% of infants as healthy or having NEC.
Conclusion: These results demonstrate the importance of HMOs and gut microbiome in preterm infant health and disease. The findings offer potential targets for biomarker development, disease risk stratification and novel avenues for supplements that may prevent life-threatening disease.
Keywords: molecular biology; oligosaccharides; prebiotic.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CS declares performing consultancy for Astarte Medical and honoraria from Danone Early Life Nutrition. NDE declares research funding from Prolacta Biosciences US and Danone Early Life Nutrition, and received lecture honoraria from Baxter and Nestle Nutrition Institute, but has no share options or other conflicts. LB is UC San Diego Chair of Collaborative Human Milk Research, endowed by the Family Larsson-Rosenquist Foundation and serves on the foundation’s scientific advisory board. LB is coinventor on patent applications regarding human milk oligosaccharides in prevention of necrotising enterocolitis and other inflammatory disorders. The other authors declare that they have no competing interests.
Figures


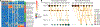

References
-
- Masi AC, Stewart CJ. The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Hum Dev 2019;138:104854. - PubMed
-
- Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
