Collaboration between patient organisations and a clinical research sponsor in a rare disease condition: learnings from a community advisory board and best practice for future collaborations
- PMID: 33328257
- PMCID: PMC7745690
- DOI: 10.1136/bmjopen-2020-039473
Collaboration between patient organisations and a clinical research sponsor in a rare disease condition: learnings from a community advisory board and best practice for future collaborations
Abstract
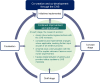
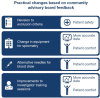
Introduction Transparent collaborations between patient organisations (POs) and clinical research sponsors (CRS) can identify and address the unmet needs of patients and caregivers. These insights can improve clinical trial participant experience and delivery of medical innovations necessary to advance health outcomes and standards of care. We share our experiences from such a collaboration undertaken surrounding the SENSCIS® clinical trial (NCT02597933), and discuss its impact during, and legacy beyond, the trial.Summary We describe the establishment of a community advisory board (CAB): a transparent, multiyear collaboration between the scleroderma patient community and a CRS. We present shared learnings from the collaboration, which is split into three main areas: (1) the implementation and conduct of the clinical trial; (2) analysis and dissemination of the results; and (3) aspects of the collaboration not related to the trial.1. The scleroderma CAB reviewed and provided advice on trial conduct and reporting. This led to the improvement and optimisation of trial procedures; meaningful, patient-focused adaptations were made to address challenges relevant to scleroderma-associated interstitial lung disease patients.2. To ensure that results of the trial were accessible to lay audiences and patients, written lay summaries were developed by the trial sponsor with valuable input from the CAB to ensure that language and figures were understandable.3. The CAB and the CRS also collaborated to co-develop opening tools for medication blister packs and bottles. In addition, to raise disease awareness among physicians, patients and caregivers, educational materials to improve diagnosis and management of scleroderma were co-created and delivered by the CAB and CRS.Conclusions This collaboration between POs and a CRS, in a rare disease condition, led to meaningful improvements in patient safety, comfort and self-management and addressed information needs. This collaboration may serve as a template of best practice for future collaborations between POs, research sponsors and other healthcare stakeholders.
Keywords: clinical trials; interstitial lung disease; medical education & training.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors have read and understood the BMJ policy on declaration of interests and have the following interests to declare:AR, MS, JW, RJR, ATK, IG, EB, CL, AGo, APPG, FH and RC did not receive any personal payments for their contributions to this article.AR, MS, JW, RJR, ATK, IG, EB, CL and APPG declare payments from Boehringer Ingelheim International GmbH to their respective POs (FESCA aisbl., Scleroderma Canada and Scleroderma Foundation, USA) for participation in community advisory board meetings and payment of travel expenses. AGo declares that Boehringer Ingelheim International GmbH paid for their travel expenses for participation in community advisory board meetings. AR, MS, JW and RJR declare that their POs (FESCA aisbl., Scleroderma Canada and Scleroderma Foundation, USA) received additional payments from Boehringer Ingelheim International GmbH for speaker and advisory services including payment of travel expenses. FH and RC declare that Boehringer Ingelheim International GmbH paid for their travel expenses for speaker and advisory services including one BI internal meeting and community advisory board meetings.RJR declares that the Scleroderma Foundation (USA) has received sponsorships in support of the annual scleroderma awareness raising campaign and the Scleroderma National Patient Education Conference from both Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals. FH and RC declare that EURORDIS-Rare Diseases Europe has received sponsorships from Boehringer Ingelheim International GmbH for the EURORDIS Round Table of Companies and the European Conference on Rare Diseases and Orphan Products. AR and ATK declare that FESCA aisbl. has received sponsorships in support of their annual scleroderma awareness raising campaign and World Scleroderma Conference from Boehringer Ingelheim International GmbH. MS declares that Scleroderma Canada has received sponsorships in support of their Scleroderma Canada Patient Conference from Boehringer Ingelheim International GmbH. These sponsorships are not related to this article. AGi has worked with the research sponsor (BI) as a paid consultant since September 2016. MG, LM, JLLF, FS, WS and HF are paid employees of the research sponsor (BI).
Figures



References
-
- US Food & Drug Administration CDER patient-focused drug development, 2019. Available: https://www.fda.gov/drugs/development-approval-process-drugs/cder-patien... [Accessed 5 Dec 2019].
-
- European Medicines Agency EMA and FDA reinforce collaboration on patient engagement, 2016. Available: https://www.ema.europa.eu/en/news/ema-fda-reinforce-collaboration-patien... [Accessed 5 Dec 2019].
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
