An Angiotensin-Responsive Connection from the Lamina Terminalis to the Paraventricular Nucleus of the Hypothalamus Evokes Vasopressin Secretion to Increase Blood Pressure in Mice
- PMID: 33328294
- PMCID: PMC7896018
- DOI: 10.1523/JNEUROSCI.1600-20.2020
An Angiotensin-Responsive Connection from the Lamina Terminalis to the Paraventricular Nucleus of the Hypothalamus Evokes Vasopressin Secretion to Increase Blood Pressure in Mice
Abstract
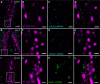
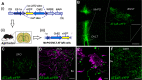
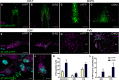
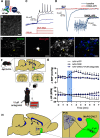
Blood pressure is controlled by endocrine, autonomic, and behavioral responses that maintain blood volume and perfusion pressure at levels optimal for survival. Although it is clear that central angiotensin type 1a receptors (AT1aR; encoded by the Agtr1a gene) influence these processes, the neuronal circuits mediating these effects are incompletely understood. The present studies characterize the structure and function of AT1aR neurons in the lamina terminalis (containing the median preoptic nucleus and organum vasculosum of the lamina terminalis), thereby evaluating their roles in blood pressure control. Using male Agtr1a-Cre mice, neuroanatomical studies reveal that AT1aR neurons in the area are largely glutamatergic and send projections to the paraventricular nucleus of the hypothalamus (PVN) that appear to synapse onto vasopressin-synthesizing neurons. To evaluate the functionality of these lamina terminalis AT1aR neurons, we virally delivered light-sensitive opsins and then optogenetically excited or inhibited the neurons while evaluating cardiovascular parameters or fluid intake. Optogenetic excitation robustly elevated blood pressure, water intake, and sodium intake, while optogenetic inhibition produced the opposite effects. Intriguingly, optogenetic excitation of these AT1aR neurons of the lamina terminalis also resulted in Fos induction in vasopressin neurons within the PVN and supraoptic nucleus. Further, within the PVN, selective optogenetic stimulation of afferents that arise from these lamina terminalis AT1aR neurons induced glutamate release onto magnocellular neurons and was sufficient to increase blood pressure. These cardiovascular effects were attenuated by systemic pretreatment with a vasopressin-1a-receptor antagonist. Collectively, these data indicate that excitation of lamina terminalis AT1aR neurons induces neuroendocrine and behavioral responses that increase blood pressure.SIGNIFICANCE STATEMENT Hypertension is a widespread health problem and risk factor for cardiovascular disease. Although treatments exist, a substantial percentage of patients suffer from "drug-resistant" hypertension, a condition associated with increased activation of brain angiotensin receptors, enhanced sympathetic nervous system activity, and elevated vasopressin levels. The present study highlights a role for angiotensin Type 1a receptor expressing neurons located within the lamina terminalis in regulating endocrine and behavioral responses that are involved in maintaining cardiovascular homeostasis. More specifically, data presented here reveal functional excitatory connections between angiotensin-sensitive neurons in the lamina terminals and vasopressin neurons in the paraventricular nucleus of the hypothalamus, and further indicate that activation of this circuit raises blood pressure. These neurons may be a promising target for antihypertensive therapeutics.
Keywords: angiotensin Type 1 receptors; blood pressure; hypertension; median preoptic nucleus; renin-angiotensin system.
Copyright © 2021 the authors.
Figures


Similar articles
-
Identification of Novel Cross-Talk between the Neuroendocrine and Autonomic Stress Axes Controlling Blood Pressure.J Neurosci. 2021 May 26;41(21):4641-4657. doi: 10.1523/JNEUROSCI.0251-21.2021. Epub 2021 Apr 15. J Neurosci. 2021. PMID: 33858944 Free PMC article.
-
A Unique "Angiotensin-Sensitive" Neuronal Population Coordinates Neuroendocrine, Cardiovascular, and Behavioral Responses to Stress.J Neurosci. 2017 Mar 29;37(13):3478-3490. doi: 10.1523/JNEUROSCI.3674-16.2017. Epub 2017 Feb 20. J Neurosci. 2017. PMID: 28219987 Free PMC article.
-
Optical perturbation of Agtr1a-containing neurons and afferents within the caudal nucleus of the solitary tract modulates sodium intake.Physiol Behav. 2024 Oct 1;284:114624. doi: 10.1016/j.physbeh.2024.114624. Epub 2024 Jul 2. Physiol Behav. 2024. PMID: 38959991
-
Preoptic–Periventricular Integrative Mechanisms Involved in Behavior, Fluid–Electrolyte Balance, and Pressor Responses.In: De Luca LA Jr, Menani JV, Johnson AK, editors. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 4. In: De Luca LA Jr, Menani JV, Johnson AK, editors. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 4. PMID: 24829988 Free Books & Documents. Review.
-
Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis.Clin Exp Pharmacol Physiol. 2001 Dec;28(12):990-2. doi: 10.1046/j.1440-1681.2001.03592.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11903300 Review.
Cited by
-
Uncovering the brain-wide pattern of synaptic input to vasopressin-expressing neurons in the paraventricular nucleus of the hypothalamus.J Comp Neurol. 2023 Jul;531(10):1017-1031. doi: 10.1002/cne.25476. Epub 2023 Apr 30. J Comp Neurol. 2023. PMID: 37121600 Free PMC article.
-
WNK1 promotes water homeostasis by acting as a central osmolality sensor for arginine vasopressin release.J Clin Invest. 2023 Jun 1;133(11):e164222. doi: 10.1172/JCI164222. J Clin Invest. 2023. PMID: 37071482 Free PMC article.
-
Identification of Novel Cross-Talk between the Neuroendocrine and Autonomic Stress Axes Controlling Blood Pressure.J Neurosci. 2021 May 26;41(21):4641-4657. doi: 10.1523/JNEUROSCI.0251-21.2021. Epub 2021 Apr 15. J Neurosci. 2021. PMID: 33858944 Free PMC article.
-
Preoptic BRS3 neurons increase body temperature and heart rate via multiple pathways.Cell Metab. 2021 Jul 6;33(7):1389-1403.e6. doi: 10.1016/j.cmet.2021.05.001. Epub 2021 May 25. Cell Metab. 2021. PMID: 34038711 Free PMC article.
-
Distinct neural representations of hunger and thirst in neonatal mice before the emergence of food- and water-seeking behaviors.Curr Biol. 2025 Jul 7;35(13):3106-3118.e4. doi: 10.1016/j.cub.2025.05.042. Epub 2025 Jun 11. Curr Biol. 2025. PMID: 40505665
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
