Zika virus is transmitted in neural progenitor cells via cell-to-cell spread and infection is inhibited by the autophagy inducer trehalose
- PMID: 33328307
- PMCID: PMC8092816
- DOI: 10.1128/JVI.02024-20
Zika virus is transmitted in neural progenitor cells via cell-to-cell spread and infection is inhibited by the autophagy inducer trehalose
Abstract
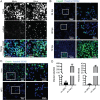
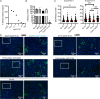
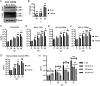
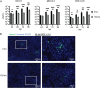
Zika virus (ZIKV) is a mosquito-borne human pathogen that causes congenital Zika syndrome and neurological symptoms in some adults. There are currently no approved treatments or vaccines for ZIKV, and exploration of therapies targeting host processes could avoid viral development of drug resistance. The purpose of our study was to determine if the non-toxic and widely used disaccharide trehalose, which showed antiviral activity against Human Cytomegalovirus (HCMV) in our previous work, could restrict ZIKV infection in clinically relevant neural progenitor cells (NPCs). Trehalose is known to induce autophagy, the degradation and recycling of cellular components. Whether autophagy is proviral or antiviral for ZIKV is controversial and depends on cell type and specific conditions used to activate or inhibit autophagy. We show here that trehalose treatment of NPCs infected with recent ZIKV isolates from Panama and Puerto Rico significantly reduces viral replication and spread. In addition, we demonstrate that ZIKV infection in NPCs spreads primarily cell-to-cell as an expanding infectious center, and NPCs are infected via contact with infected cells far more efficiently than by cell-free virus. Importantly, ZIKV was able to spread in NPCs in the presence of neutralizing antibody.Importance Zika virus causes birth defects and can lead to neurological disease in adults. While infection rates are currently low, ZIKV remains a public health concern with no treatment or vaccine available. Targeting a cellular pathway to inhibit viral replication is a potential treatment strategy that avoids development of antiviral resistance. We demonstrate in this study that the non-toxic autophagy-inducing disaccharide trehalose reduces spread and output of ZIKV in infected neural progenitor cells (NPCs), the major cells infected in the fetus. We show that ZIKV spreads cell-to-cell in NPCs as an infectious center and that NPCs are more permissive to infection by contact with infected cells than by cell-free virus. We find that neutralizing antibody does not prevent the spread of the infection in NPCs. These results are significant in demonstrating anti-ZIKV activity of trehalose and in clarifying the primary means of Zika virus spread in clinically relevant target cells.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
Heparin Protects Human Neural Progenitor Cells from Zika Virus-Induced Cell Death While Preserving Their Differentiation into Mature Neuroglial Cells.J Virol. 2022 Oct 12;96(19):e0112222. doi: 10.1128/jvi.01122-22. Epub 2022 Sep 19. J Virol. 2022. PMID: 36121298 Free PMC article.
-
Trehalose, an mTOR-Independent Inducer of Autophagy, Inhibits Human Cytomegalovirus Infection in Multiple Cell Types.J Virol. 2015 Nov 11;90(3):1259-77. doi: 10.1128/JVI.02651-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26559848 Free PMC article.
-
The Compound SBI-0090799 Inhibits Zika Virus Infection by Blocking De Novo Formation of the Membranous Replication Compartment.J Virol. 2021 Oct 27;95(22):e0099621. doi: 10.1128/JVI.00996-21. Epub 2021 Sep 1. J Virol. 2021. PMID: 34468177 Free PMC article.
-
Congenital Abnormalities: Consequence of Maternal Zika Virus Infection: A Narrative Review.Infect Disord Drug Targets. 2017;17(1):3-13. doi: 10.2174/1871526516666161018104916. Infect Disord Drug Targets. 2017. PMID: 27758685 Review.
-
Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication.Int J Mol Sci. 2019 Feb 28;20(5):1048. doi: 10.3390/ijms20051048. Int J Mol Sci. 2019. PMID: 30823365 Free PMC article. Review.
Cited by
-
Zika Virus NS1 Drives Tunneling Nanotube Formation for Mitochondrial Transfer, Enhanced Survival, Interferon Evasion, and Stealth Transmission in Trophoblasts.Res Sq [Preprint]. 2023 Dec 6:rs.3.rs-3674059. doi: 10.21203/rs.3.rs-3674059/v1. Res Sq. 2023. Update in: Nat Commun. 2025 Feb 20;16(1):1803. doi: 10.1038/s41467-025-56927-2. PMID: 38106210 Free PMC article. Updated. Preprint.
-
Heparin Protects Human Neural Progenitor Cells from Zika Virus-Induced Cell Death While Preserving Their Differentiation into Mature Neuroglial Cells.J Virol. 2022 Oct 12;96(19):e0112222. doi: 10.1128/jvi.01122-22. Epub 2022 Sep 19. J Virol. 2022. PMID: 36121298 Free PMC article.
-
Trehalose supports the growth of Aedes aegypti cells and modifies gene expression and dengue virus replication.bioRxiv [Preprint]. 2024 Dec 4:2024.12.03.626538. doi: 10.1101/2024.12.03.626538. bioRxiv. 2024. Update in: PLoS Pathog. 2025 May 6;21(5):e1012795. doi: 10.1371/journal.ppat.1012795. PMID: 39677712 Free PMC article. Updated. Preprint.
-
Trehalose supports the growth of Aedes aegypti cells and modifies gene expression and dengue virus type 2 replication.PLoS Pathog. 2025 May 6;21(5):e1012795. doi: 10.1371/journal.ppat.1012795. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40327709 Free PMC article.
-
Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts.Nat Commun. 2025 Feb 20;16(1):1803. doi: 10.1038/s41467-025-56927-2. Nat Commun. 2025. PMID: 39979240 Free PMC article.
References
-
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi:10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
-
- Chibueze EC, Tirado V, Lopes KS, Balogun OO, Takemoto Y, Swa T, Dagvadorj A, Nagata C, Morisaki N, Menendez C, Ota E, Mori R, Oladapo OT. 2017. Zika virus infection in pregnancy: a systematic review of disease course and complications. Reprod Health 14:28. doi:10.1186/s12978-017-0285-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

