Eosinophils improve cardiac function after myocardial infarction
- PMID: 33328477
- PMCID: PMC7745020
- DOI: 10.1038/s41467-020-19297-5
Eosinophils improve cardiac function after myocardial infarction
Abstract
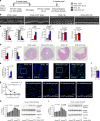
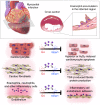
Clinical studies reveal changes in blood eosinophil counts and eosinophil cationic proteins that may serve as risk factors for human coronary heart diseases. Here we report an increase of blood or heart eosinophil counts in humans and mice after myocardial infarction (MI), mostly in the infarct region. Genetic or inducible depletion of eosinophils exacerbates cardiac dysfunction, cell death, and fibrosis post-MI, with concurrent acute increase of heart and chronic increase of splenic neutrophils and monocytes. Mechanistic studies reveal roles of eosinophil IL4 and cationic protein mEar1 in blocking H2O2- and hypoxia-induced mouse and human cardiomyocyte death, TGF-β-induced cardiac fibroblast Smad2/3 activation, and TNF-α-induced neutrophil adhesion on the heart endothelial cell monolayer. In vitro-cultured eosinophils from WT mice or recombinant mEar1 protein, but not eosinophils from IL4-deficient mice, effectively correct exacerbated cardiac dysfunctions in eosinophil-deficient ∆dblGATA mice. This study establishes a cardioprotective role of eosinophils in post-MI hearts.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
A protective role for eosinophils in MI.Nat Rev Cardiol. 2021 Apr;18(4):230. doi: 10.1038/s41569-021-00507-6. Nat Rev Cardiol. 2021. PMID: 33437041 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

