Neutralizing the pathological effects of extracellular histones with small polyanions
- PMID: 33328478
- PMCID: PMC7744542
- DOI: 10.1038/s41467-020-20231-y
Neutralizing the pathological effects of extracellular histones with small polyanions
Abstract
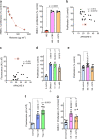
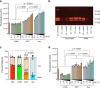
Extracellular histones in neutrophil extracellular traps (NETs) or in chromatin from injured tissues are highly pathological, particularly when liberated by DNases. We report the development of small polyanions (SPAs) (~0.9-1.4 kDa) that interact electrostatically with histones, neutralizing their pathological effects. In vitro, SPAs inhibited the cytotoxic, platelet-activating and erythrocyte-damaging effects of histones, mechanistic studies revealing that SPAs block disruption of lipid-bilayers by histones. In vivo, SPAs significantly inhibited sepsis, deep-vein thrombosis, and cardiac and tissue-flap models of ischemia-reperfusion injury (IRI), but appeared to differ in their capacity to neutralize NET-bound versus free histones. Analysis of sera from sepsis and cardiac IRI patients supported these differential findings. Further investigations revealed this effect was likely due to the ability of certain SPAs to displace histones from NETs, thus destabilising the structure. Finally, based on our work, a non-toxic SPA that inhibits both NET-bound and free histone mediated pathologies was identified for clinical development.
Conflict of interest statement
C.R.P., C.H.O., L.A.C., F.K., B.J.C.Q., A.B., A.M.B., C.F., R.W.S., and M.v.I. have filed patent applications covering the use of SPAs as inhibitors of sepsis. All other authors declare no competing interests.
Figures

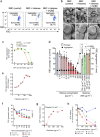
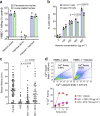
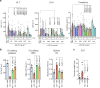
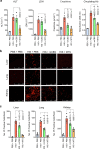

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

