Implementation of a 4Pi-SMS super-resolution microscope
- PMID: 33328610
- PMCID: PMC9118368
- DOI: 10.1038/s41596-020-00428-7
Implementation of a 4Pi-SMS super-resolution microscope
Abstract
The development of single-molecule switching (SMS) fluorescence microscopy (also called single-molecule localization microscopy) over the last decade has enabled researchers to image cell biological structures at unprecedented resolution. Using two opposing objectives in a so-called 4Pi geometry doubles the available numerical aperture, and coupling this with interferometric detection has demonstrated 3D resolution down to 10 nm over entire cellular volumes. The aim of this protocol is to enable interested researchers to establish 4Pi-SMS super-resolution microscopy in their laboratories. We describe in detail how to assemble the optomechanical components of a 4Pi-SMS instrument, align its optical beampath and test its performance. The protocol further provides instructions on how to prepare test samples of fluorescent beads, operate this instrument to acquire images of whole cells and analyze the raw image data to reconstruct super-resolution 3D data sets. Furthermore, we provide a troubleshooting guide and present examples of anticipated results. An experienced optical instrument builder will require ~12 months from the start of ordering hardware components to acquiring high-quality biological images.
Conflict of interest statement
Competing interests
J.B. has financial interests in Bruker Corp. and Hamamatsu Photonics. J.B. is co-inventor of a US patent application (US20170251191A1) related to the 4Pi-SMS system and image analysis used in this work. Y.Z. and J.B. have filed a US patent application about the salvaged fluorescence multicolor imaging method described in this work.
Figures

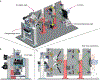

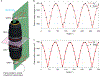

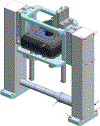
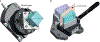
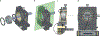

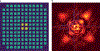


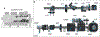







References
-
- Aquino D et al. Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores. Nat. Methods 8, 353–359 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
- 203285/C/16/Z/Wellcome Trust (Wellcome)
- 141/144/Oxford University | John Fell Fund, University of Oxford (John Fell OUP Research Fund)
- 107457/Z/15/Z/Wellcome Trust (Wellcome)
- R01 GM118486/GM/NIGMS NIH HHS/United States
- U01 EB021223/EB/NIBIB NIH HHS/United States
- T32 EB019941/EB/NIBIB NIH HHS/United States
- 095927/A/11/Z/Wellcome Trust (Wellcome)
- WT_/Wellcome Trust/United Kingdom
- 095927/B/11/Z/Wellcome Trust (Wellcome)
- 203144/Z/16/Z/Wellcome Trust (Wellcome)
- 203285/B/16/Z/Wellcome Trust (Wellcome)
- 105605/Z/14/Z/Wellcome Trust (Wellcome)
- 203285/Z/16/Z/Wellcome Trust (Wellcome)
- EIPOD/European Molecular Biology Laboratory (EMBL Heidelberg)
LinkOut - more resources
Full Text Sources

