Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis
- PMID: 33328637
- PMCID: PMC7612440
- DOI: 10.1038/s41586-020-03046-1
Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis
Abstract
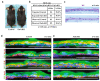
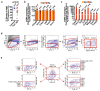
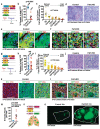
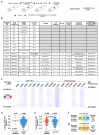
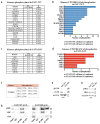
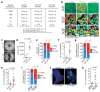
FAT1, which encodes a protocadherin, is one of the most frequently mutated genes in human cancers1-5. However, the role and the molecular mechanisms by which FAT1 mutations control tumour initiation and progression are poorly understood. Here, using mouse models of skin squamous cell carcinoma and lung tumours, we found that deletion of Fat1 accelerates tumour initiation and malignant progression and promotes a hybrid epithelial-to-mesenchymal transition (EMT) phenotype. We also found this hybrid EMT state in FAT1-mutated human squamous cell carcinomas. Skin squamous cell carcinomas in which Fat1 was deleted presented increased tumour stemness and spontaneous metastasis. We performed transcriptional and chromatin profiling combined with proteomic analyses and mechanistic studies, which revealed that loss of function of FAT1 activates a CAMK2-CD44-SRC axis that promotes YAP1 nuclear translocation and ZEB1 expression that stimulates the mesenchymal state. This loss of function also inactivates EZH2, promoting SOX2 expression, which sustains the epithelial state. Our comprehensive analysis identified drug resistance and vulnerabilities in FAT1-deficient tumours, which have important implications for cancer therapy. Our studies reveal that, in mouse and human squamous cell carcinoma, loss of function of FAT1 promotes tumour initiation, progression, invasiveness, stemness and metastasis through the induction of a hybrid EMT state.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

