Single-Cell Visualization Deep in Brain Structures by Gene Transfer
- PMID: 33328900
- PMCID: PMC7710941
- DOI: 10.3389/fncir.2020.586043
Single-Cell Visualization Deep in Brain Structures by Gene Transfer
Abstract
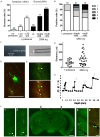
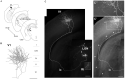
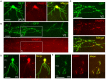
A projection neuron targets multiple regions beyond the functional brain area. In order to map neuronal connectivity in a massive neural network, a means for visualizing the entire morphology of a single neuron is needed. Progress has facilitated single-neuron analysis in the cerebral cortex, but individual neurons in deep brain structures remain difficult to visualize. To this end, we developed an in vivo single-cell electroporation method for juvenile and adult brains that can be performed under a standard stereomicroscope. This technique involves rapid gene transfection and allows the visualization of dendritic and axonal morphologies of individual neurons located deep in brain structures. The transfection efficiency was enhanced by directly injecting the expression vector encoding green fluorescent protein instead of monitoring cell attachment to the electrode tip. We obtained similar transfection efficiencies in both young adult (≥P40) and juvenile mice (P21-30). By tracing the axons of thalamocortical neurons, we identified a specific subtype of neuron distinguished by its projection pattern. Additionally, transfected mOrange-tagged vesicle-associated membrane protein 2-a presynaptic protein-was strongly localized in terminal boutons of thalamocortical neurons. Thus, our in vivo single-cell gene transfer system offers rapid single-neuron analysis deep in brain. Our approach combines observation of neuronal morphology with functional analysis of genes of interest, which can be useful for monitoring changes in neuronal activity corresponding to specific behaviors in living animals.
Keywords: lateral pulvinar; overexpression; postnatal development; presynaptic protein; single-cell electroporation; thalamus; visual cortex.
Copyright © 2020 Sugiyama, Sugi, Iijima and Hou.
Figures



Similar articles
-
Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex.Eur J Neurosci. 2010 Feb;31(3):410-24. doi: 10.1111/j.1460-9568.2009.07070.x. Epub 2010 Jan 25. Eur J Neurosci. 2010. PMID: 20105242
-
In vivo electroporation to physiologically identified deep brain regions in postnatal mammals.Brain Struct Funct. 2015;220(3):1307-16. doi: 10.1007/s00429-014-0724-x. Epub 2014 Feb 14. Brain Struct Funct. 2015. PMID: 24526275
-
Cortical Divergent Projections in Mice Originate from Two Sequentially Generated, Distinct Populations of Excitatory Cortical Neurons with Different Initial Axonal Outgrowth Characteristics.Cereb Cortex. 2016 May;26(5):2257-2270. doi: 10.1093/cercor/bhv077. Epub 2015 Apr 16. Cereb Cortex. 2016. PMID: 25882037
-
Diverse axonal morphologies of individual callosal projection neurons reveal new insights into brain connectivity.Curr Opin Neurobiol. 2024 Feb;84:102837. doi: 10.1016/j.conb.2023.102837. Epub 2024 Jan 24. Curr Opin Neurobiol. 2024. PMID: 38271848 Free PMC article. Review.
-
Inputs from the thalamocortical system on axon pathfinding mechanisms.Curr Opin Neurobiol. 2014 Aug;27:143-50. doi: 10.1016/j.conb.2014.03.013. Epub 2014 Apr 17. Curr Opin Neurobiol. 2014. PMID: 24742382 Review.
Cited by
-
Targeted approaches to delineate neuronal morphology during early development.Front Cell Neurosci. 2023 Oct 3;17:1259360. doi: 10.3389/fncel.2023.1259360. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37854514 Free PMC article. Review.
-
Single-Cell Labeling Strategies to Dissect Neuronal Structures and Local Functions.Biology (Basel). 2023 Feb 16;12(2):321. doi: 10.3390/biology12020321. Biology (Basel). 2023. PMID: 36829594 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

