Mixture Coding and Segmentation in the Anterior Piriform Cortex
- PMID: 33328914
- PMCID: PMC7710992
- DOI: 10.3389/fnsys.2020.604718
Mixture Coding and Segmentation in the Anterior Piriform Cortex
Abstract
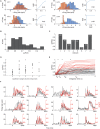
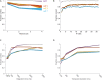
Coding of odorous stimuli has been mostly studied using single isolated stimuli. However, a single sniff of air in a natural environment is likely to introduce airborne chemicals emitted by multiple objects into the nose. The olfactory system is therefore faced with the task of segmenting odor mixtures to identify objects in the presence of rich and often unpredictable backgrounds. The piriform cortex is thought to be the site of object recognition and scene segmentation, yet the nature of its responses to odorant mixtures is largely unknown. In this study, we asked two related questions. (1) How are mixtures represented in the piriform cortex? And (2) Can the identity of individual mixture components be read out from mixture representations in the piriform cortex? To answer these questions, we recorded single unit activity in the piriform cortex of naïve mice while sequentially presenting single odorants and their mixtures. We find that a normalization model explains mixture responses well, both at the single neuron, and at the population level. Additionally, we show that mixture components can be identified from piriform cortical activity by pooling responses of a small population of neurons-in many cases a single neuron is sufficient. These results indicate that piriform cortical representations are well suited to perform figure-background segmentation without the need for learning.
Keywords: figure-background; normalization; odor; olfaction; smell.
Copyright © 2020 Penker, Licht, Hofer and Rokni.
Figures



References
LinkOut - more resources
Full Text Sources

