Cannabinoids, Inner Ear, Hearing, and Tinnitus: A Neuroimmunological Perspective
- PMID: 33329293
- PMCID: PMC7719758
- DOI: 10.3389/fneur.2020.505995
Cannabinoids, Inner Ear, Hearing, and Tinnitus: A Neuroimmunological Perspective
Abstract
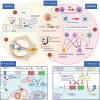
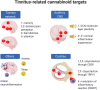
Cannabis has been used for centuries for recreational and therapeutic purposes. Whereas, the recreative uses are based on the psychotropic effect of some of its compounds, its therapeutic effects range over a wide spectrum of actions, most of which target the brain or the immune system. Several studies have found cannabinoid receptors in the auditory system, both at peripheral and central levels, thus raising the interest in cannabinoid signaling in hearing, and especially in tinnitus, which is affected also by anxiety, memory, and attention circuits where cannabinoid effects are well described. Available studies on animal models of tinnitus suggest that cannabinoids are not likely to be helpful in tinnitus treatment and could even be harmful. However, the pharmacology of cannabinoids is very complex, and most studies focused on neural CB1R-based responses. Cannabinoid effects on the immune system (where CB2Rs predominate) are increasingly recognized as essential in understanding nervous system pathological responses, and data on immune cannabinoid targets have emerged in the auditory system as well. In addition, nonclassical cannabinoid targets (such as TRP channels) appear to play an important role in the auditory system as well. This review will focus on neuroimmunological mechanisms for cannabinoid effects and their possible use as protective and therapeutic agents in the ear and auditory system, especially in tinnitus.
Keywords: auditory; cannabinoids; hearing; neuroimmune; tinnitus.
Copyright © 2020 Perin, Mabou Tagne, Enrico, Marino, Cosentino, Pizzala and Boselli.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

