Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management
- PMID: 33329350
- PMCID: PMC7734299
- DOI: 10.3389/fneur.2020.596981
Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management
Abstract
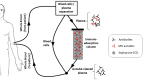
Myasthenia gravis (MG) is the most common autoimmune disorder affecting the neuromuscular junction, characterized by skeletal muscle weakness and fatigability. It is caused by autoantibodies targeting proteins of the neuromuscular junction; ~85% of MG patients have autoantibodies against the muscle acetylcholine receptor (AChR-MG), whereas about 5% of MG patients have autoantibodies against the muscle specific kinase (MuSK-MG). In the remaining about 10% of patients no autoantibodies can be found with the classical diagnostics for AChR and MuSK antibodies (seronegative MG, SN-MG). Since serological tests are relatively easy and non-invasive for disease diagnosis, the improvement of methods for the detection of known autoantibodies or the discovery of novel autoantibody specificities to diminish SN-MG and to facilitate differential diagnosis of similar diseases, is crucial. Radioimmunoprecipitation assays (RIPA) are the staple for MG antibody detection, but over the past years, using cell-based assays (CBAs) or improved highly sensitive RIPAs, it has been possible to detect autoantibodies in previously SN-MG patients. This led to the identification of more patients with antibodies to the classical antigens AChR and MuSK and to the third MG autoantigen, the low-density lipoprotein receptor-related protein 4 (LRP4), while antibodies against other extracellular or intracellular targets, such as agrin, Kv1.4 potassium channels, collagen Q, titin, the ryanodine receptor and cortactin have been found in some MG patients. Since the autoantigen targeted determines in part the clinical manifestations, prognosis and response to treatment, serological tests are not only indispensable for initial diagnosis, but also for monitoring treatment efficacy. Importantly, knowing the autoantibody profile of MG patients could allow for more efficient personalized therapeutic approaches. Significant progress has been made over the past years toward the development of antigen-specific therapies, targeting only the specific immune cells or autoantibodies involved in the autoimmune response. In this review, we will present the progress made toward the development of novel sensitive autoantibody detection assays, the identification of new MG autoantigens, and the implications for improved antigen-specific therapeutics. These advancements increase our understanding of MG pathology and improve patient quality of life by providing faster, more accurate diagnosis and better disease management.
Keywords: LRP4; MuSK; acetylcholine receptor; autoantibody; autoimmunity; diagnosis; myasthenia gravis; therapy.
Copyright © 2020 Lazaridis and Tzartos.
Conflict of interest statement
ST has shares in the research and diagnostic laboratory Tzartos NeuroDiagnostics. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Verschuuren JJ, Huijbers MG, Plomp JJ, Niks EH, Molenaar PC, Martinez-Martinez P, et al. . Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev. (2013) 12:918–23. 10.1016/j.autrev.2013.03.001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

