Alpha 1 Antitrypsin-Deficient Macrophages Have Impaired Efferocytosis of Apoptotic Neutrophils
- PMID: 33329539
- PMCID: PMC7714766
- DOI: 10.3389/fimmu.2020.574410
Alpha 1 Antitrypsin-Deficient Macrophages Have Impaired Efferocytosis of Apoptotic Neutrophils
Abstract
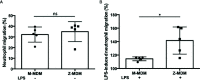
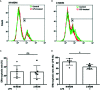
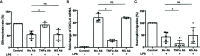
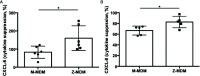
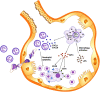
Alpha 1 antitrypsin deficiency (AATD) is an autosomal co-dominant disorder characterized by a low level of circulating AAT, which significantly reduces protection for the lower airways against proteolytic burden caused by neutrophils. Neutrophils, which are terminally differentiated innate immune cells and play a critical role to clear pathogens, accumulate excessively in the lung of AATD individuals. The neutrophil burden in AATD individuals increases the risk for early-onset destructive lung diseases by producing neutrophil products such as reactive oxygen radicals and various proteases. The level of AAT in AATD individuals is not sufficient to inhibit the activity of neutrophil chemotactic factors such as CXCL-8 and LTB4, which could lead to alveolar neutrophil accumulation in AATD individuals. However, as neutrophils have a short lifespan, and apoptotic neutrophils are rapidly cleared by alveolar macrophages that outnumber the apoptotic neutrophils in the pulmonary alveolus, the increased chemotaxis activity does not fully explain the persistent neutrophil accumulation and the resulting chronic inflammation in AATD individuals. Here, we propose that the ability of alveolar macrophages to clear apoptotic neutrophils is impaired in AATD individuals and it could be the main driver to cause neutrophil accumulation in their lung. This study demonstrates that Z-AAT variant significantly increases the expression of pro-inflammatory cytokines including CXCL-8, CXCL1, LTB4, and TNFα in LPS-treated macrophages. These cytokines play a central role in neutrophil recruitment to the lung and in clearance of apoptotic neutrophils by macrophages. Our result shows that LPS treatment significantly reduces the efferocytosis ability of macrophages with the Z-AAT allele by inducing TNFα expression. We incubated monocyte-derived macrophages (MDMs) with apoptotic neutrophils and found that after 3 h of co-incubation, the expression level of CXCL-8 is reduced in M-MDMs but increased in Z-MDMs. This result shows that the expression of inflammatory cytokines could be increased by impaired efferocytosis. It indicates that the efferocytosis ability of macrophages plays an important role in regulating cytokine expression and resolving inflammation. Findings from this study would help us better understand the multifaceted effect of AAT on regulating neutrophil balance in the lung and the underlying mechanisms.
Keywords: AAT deficiency; Alpha 1 antitrysin; cytokine; efferocytosis; macrophage; neutrophil.
Copyright © 2020 Lee, Lu, Oshins, West, Moneypenny, Han and Brantly.
Figures




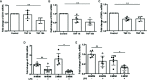
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

