Baseline Gut Microbiota Composition Is Associated With Schistosoma mansoni Infection Burden in Rodent Models
- PMID: 33329584
- PMCID: PMC7718013
- DOI: 10.3389/fimmu.2020.593838
Baseline Gut Microbiota Composition Is Associated With Schistosoma mansoni Infection Burden in Rodent Models
Abstract
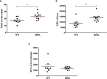
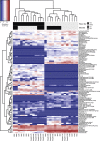
In spite of growing evidence supporting the occurrence of complex interactions between Schistosoma and gut bacteria in mice and humans, no data is yet available on whether worm-mediated changes in microbiota composition are dependent on the baseline gut microbial profile of the vertebrate host. In addition, the impact of such changes on the susceptibility to, and pathophysiology of, schistosomiasis remains largely unexplored. In this study, mice colonized with gut microbial populations from a human donor (HMA mice), as well as microbiota-wild type (WT) animals, were infected with Schistosoma mansoni, and alterations of their gut microbial profiles at 50 days post-infection were compared to those occurring in uninfected HMA and WT rodents, respectively. Significantly higher worm and egg burdens, together with increased specific antibody responses to parasite antigens, were observed in HMA compared to WT mice. These differences were associated to extensive dissimilarities between the gut microbial profiles of each HMA and WT groups of mice at baseline; in particular, the gut microbiota of HMA animals was characterized by low microbial alpha diversity and expanded Proteobacteria, as well as by the absence of putative immunomodulatory bacteria (e.g. Lactobacillus). Furthermore, differences in infection-associated changes in gut microbiota composition were observed between HMA and WT mice. Altogether, our findings support the hypothesis that susceptibility to S.mansoni infection in mice is partially dependent on the composition of the host baseline microbiota. Moreover, this study highlights the applicability of HMA mouse models to address key biological questions on host-parasite-microbiota relationships in human helminthiases.
Keywords: Schistosoma mansoni; dysbiosis; gut microbial diversity; helminth-gut microbiota interactions; human-microbiota associated mouse models; immune-modulation.
Copyright © 2020 Cortés, Clare, Costain, Almeida, McCarthy, Harcourt, Brandt, Tolley, Rooney, Berriman, Lawley, MacDonald, Rinaldi and Cantacessi.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

