Xeroderma Pigmentosum C (XPC) Mutations in Primary Fibroblasts Impair Base Excision Repair Pathway and Increase Oxidative DNA Damage
- PMID: 33329698
- PMCID: PMC7728722
- DOI: 10.3389/fgene.2020.561687
Xeroderma Pigmentosum C (XPC) Mutations in Primary Fibroblasts Impair Base Excision Repair Pathway and Increase Oxidative DNA Damage
Abstract
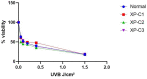
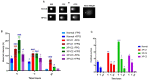
Xeroderma Pigmentosum C (XPC) is a multi-functional protein that is involved not only in the repair of bulky lesions, post-irradiation, via nucleotide excision repair (NER) per se but also in oxidative DNA damage mending. Since base excision repair (BER) is the primary regulator of oxidative DNA damage, we characterized, post-Ultraviolet B-rays (UVB)-irradiation, the detailed effect of three different XPC mutations in primary fibroblasts derived from XP-C patients on mRNA, protein expression and activity of different BER factors. We found that XP-C fibroblasts are characterized by downregulated expression of different BER factors including OGG1, MYH, APE1, LIG3, XRCC1, and Polβ. Such a downregulation was also observed at OGG1, MYH, and APE1 protein levels. This was accompanied with an increase in DNA oxidative lesions, as evidenced by 8-oxoguanine levels, immediately post-UVB-irradiation. Unlike in normal control cells, these oxidative lesions persisted over time in XP-C cells having lower excision repair capacities. Taken together, our results indicated that an impaired BER pathway in XP-C fibroblasts leads to longer persistence and delayed repair of oxidative DNA damage. This might explain the diverse clinical phenotypes in XP-C patients suffering from cancer in both photo-protected and photo-exposed areas. Therapeutic strategies based on reinforcement of BER pathway might therefore represent an innovative path for limiting the drawbacks of NER-based diseases, as in XP-C case.
Keywords: Xeroderma Pigmentosum C; base excision repair; nucleotide excision repair; oxidative DNA damage; oxidative stress; skin cancer; ultra violet (UV) light.
Copyright © 2020 Fayyad, Kobaisi, Beal, Mahfouf, Ged, Morice-Picard, Fayyad-Kazan, Fayyad-Kazan, Badran, Rezvani and Rachidi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Berg R. J., Ruven H. J., Sands A. T., De Gruijl F. R., Mullenders L. H. (1998). Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J. Invest. Dermatol. 110 405–409. 10.1111/j.1523-1747.1998.00173.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

