microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis
- PMID: 33330058
- PMCID: PMC7729128
- DOI: 10.3389/fonc.2020.581007
microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis
Abstract
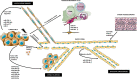
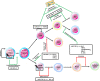
microRNAs (miRNAs) are small non-coding RNA molecules, evolutionary conserved. They target more than one mRNAs, thus influencing multiple molecular pathways, but also mRNAs may bind to a variety of miRNAs, either simultaneously or in a context-dependent manner. miRNAs biogenesis, including miRNA transcription, processing by Drosha and Dicer, transportation, RISC biding, and miRNA decay, are finely controlled in space and time. miRNAs are critical regulators in various biological processes, such as differentiation, proliferation, apoptosis, and development in both health and disease. Their dysregulation is involved in tumor initiation and progression. In tumors, they can act as onco-miRNAs or oncosuppressor-miRNA participating in distinct cellular pathways, and the same miRNA can perform both activities depending on the context. In tumor progression, the angiogenic switch is fundamental. miRNAs derived from tumor cells, endothelial cells, and cells of the surrounding microenvironment regulate tumor angiogenesis, acting as pro-angiomiR or anti-angiomiR. In this review, we described miRNA biogenesis and function, and we update the non-classical aspects of them. The most recent role in the nucleus, as transcriptional gene regulators and the different mechanisms by which they could be dysregulated, in tumor initiation and progression, are treated. In particular, we describe the role of miRNAs in sprouting angiogenesis, vessel co-option, and vasculogenic mimicry. The role of miRNAs in lymphoma angiogenesis is also discussed despite the scarcity of data. The information presented in this review reveals the need to do much more to discover the complete miRNA network regulating angiogenesis, not only using high-throughput computational analysis approaches but also morphological ones.
Keywords: angiogenesis; lymphoma; microRNA; microenvironment; tumor progression.
Copyright © 2020 Annese, Tamma, De Giorgis and Ribatti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

