Terminal Respiratory Oxidases: A Targetables Vulnerability of Mycobacterial Bioenergetics?
- PMID: 33330134
- PMCID: PMC7719681
- DOI: 10.3389/fcimb.2020.589318
Terminal Respiratory Oxidases: A Targetables Vulnerability of Mycobacterial Bioenergetics?
Abstract
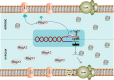
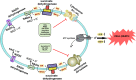
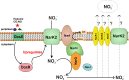
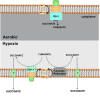
Recently, ATP synthase inhibitor Bedaquiline was approved for the treatment of multi-drug resistant tuberculosis emphasizing the importance of oxidative phosphorylation for the survival of mycobacteria. ATP synthesis is primarily dependent on the generation of proton motive force through the electron transport chain in mycobacteria. The mycobacterial electron transport chain utilizes two terminal oxidases for the reduction of oxygen, namely the bc1-aa3 supercomplex and the cytochrome bd oxidase. The bc1-aa3 supercomplex is an energy-efficient terminal oxidase that pumps out four vectoral protons, besides consuming four scalar protons during the transfer of electrons from menaquinone to molecular oxygen. In the past few years, several inhibitors of bc1-aa3 supercomplex have been developed, out of which, Q203 belonging to the class of imidazopyridine, has moved to clinical trials. Recently, the crystal structure of the mycobacterial cytochrome bc1-aa3 supercomplex was solved, providing details of the route of transfer of electrons from menaquinone to molecular oxygen. Besides providing insights into the molecular functioning, crystal structure is aiding in the targeted drug development. On the other hand, the second respiratory terminal oxidase of the mycobacterial respiratory chain, cytochrome bd oxidase, does not pump out the vectoral protons and is energetically less efficient. However, it can detoxify the reactive oxygen species and facilitate mycobacterial survival during a multitude of stresses. Quinolone derivatives (CK-2-63) and quinone derivative (Aurachin D) inhibit cytochrome bd oxidase. Notably, ablation of both the two terminal oxidases simultaneously through genetic methods or pharmacological inhibition leads to the rapid death of the mycobacterial cells. Thus, terminal oxidases have emerged as important drug targets. In this review, we have described the current understanding of the functioning of these two oxidases, their physiological relevance to mycobacteria, and their inhibitors. Besides these, we also describe the alternative terminal complexes that are used by mycobacteria to maintain energized membrane during hypoxia and anaerobic conditions.
Keywords: Aurachin D; Mycobacterium; Q203; bc1-aa3 supercomplex; cytochrome bd oxidase; electron transport chain; oxidative phoshorylation; respiratory inhibitors.
Copyright © 2020 Bajeli, Baid, Kaur, Pawar, Chaudhari and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

