Comparative Analysis of Platelet-rich Plasma Effect on Tenocytes from Normal Human Rotator Cuff Tendon and Human Rotator Cuff Tendon with Degenerative Tears
- PMID: 33330145
- PMCID: PMC7726368
- DOI: 10.5397/cise.2018.21.1.3
Comparative Analysis of Platelet-rich Plasma Effect on Tenocytes from Normal Human Rotator Cuff Tendon and Human Rotator Cuff Tendon with Degenerative Tears
Abstract
Background: Platelet-rich plasma (PRP) stimulates cell proliferation and enhances matrix gene expression and synthesis. However, there have been no comparative study of the PRP effect on the normal and degenerative tenocytes. The purpose of this study was to compare the effect of PRP on tenocytes from normal and degenerative tendon.
Methods: Tendon tissues were obtained from patients undergoing arthroscopic repair (n=9) and from healthy donors (n=3). Tenocytes were cultured with 10% (vol/vol) platelet-poor plasma, PRP activated with calcium, and PRP activated with calcium and thrombin. The total cell number was assessed at days 7 and 14. The expressions of type I and III collagen, decorin, tenascin-C, and scleraxis were evaluated by quantitative real-time reverse transcriptase polymerase chain reaction. The total collagen and glycosaminoglycan (GAG) synthesis was evaluated at days 7 and 14.
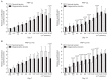
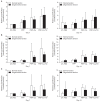
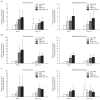
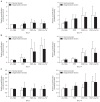
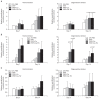
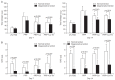
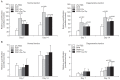
Results: No differences were observed between the groups at day 7, but cell proliferation was remarkably increased in tenocytes from the degenerative tendon at day 14. In both tenocyte groups, the gene expressions of type I and III collagen were up-regulated. GAG synthesis was greater in the normal tendon, whereas the expressions of decorin and tenascin-C were increased in tenocytes from the degenerative tendon. Tenocytes from the degenerative tendon had higher fold-change of GAG synthesis and a lower collagen III/I ratio than normal tenocytes.
Conclusions: PRP promoted the cell proliferation and enhanced the synthesis of tendon matrix in both groups. PRP has a greater positive effect on cell proliferation, matrix gene expression and synthesis in tenocytes from degenerative tendon.
Keywords: Platelet-rich plasma; Rotator cuff; Tendon; Tenocytes.
Copyright © 2018 Korean Shoulder and Elbow Society.
Conflict of interest statement
Conflict of interest None.
Figures
References
-
- Jo CH, Shin WH, Park JW, Shin JS, Kim JE. Degree of tendon degeneration and stage of rotator cuff disease. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2100–8. - PubMed
-
- Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–95. - PubMed
-
- Chaudhury S, Carr AJ. Lessons we can learn from gene expression patterns in rotator cuff tears and tendinopathies. J Shoulder Elbow Surg. 2012;21(2):191–9. - PubMed
-
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15(4):241–51. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials