Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries
- PMID: 33330352
- PMCID: PMC7672033
- DOI: 10.3389/fchem.2020.546728
Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries
Abstract
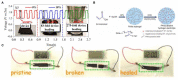
On account of high energy density depending on the utilized zinc metal anode of high theoretical capacity and its excellent security due to aqueous electrolytes that usually be locked in polymer hosts referred to as hydrogels, quasi-solid zinc-based batteries have been subjected to more and more interest from researchers. The good water retention and electrolyte load capacity of the hydrogel, contributing to the acquirement of high ionic conductivity and durability of the as-obtained quasi-solid electrolyte, play a significant role on the performance of the devices. Moreover, the chemistry of hydrogels can be tuned to endow quasi-solid electrolytes with additional functions in terms of application scenarios of solid-state batteries. Herein, the frontier disciplines of hydrogel electrolytes for Zn-based batteries were reviewed. The cross-linking process of the polymer networks for hydrogel materials with different functions, such as stretchability, compressibility, and self-healing, were also discussed to analyze the properties of the polymer electrolyte. Based on the merits of the functionalized hydrogel, the further application of hydrogel electrolytes in Zn-based batteries is the focus of this paper. The electrochemical performance and mechanical property of Zn-based batteries with functionalized hydrogel electrolytes under extreme conditions were presented to evaluate the crucial role of the polymer hydrogel electrolyte. Finally, the challenges of hydrogel electrolytes for currently developed Zn-based batteries are highlighted with the hope to boost their commercial application in energy conversion devices.
Keywords: electrolytes; hydrogel; self-healing; stretchability; zinc-based batteries.
Copyright © 2020 Lu, Jiang, Hu and Wu.
Figures


References
-
- Huang Y., Li Z., Pei Z., Liu Z., Li H., Zhu M., et al. . (2018a). Solid-state rechargeable Zn//NiCo and Zn-Air batteries with ultralong lifetime and high capacity: The role of a sodium polyacrylate hydrogel electrolyte. Adv. Energy Mater. 8:1802288. 10.1002/aenm.201802288 - DOI
Publication types
LinkOut - more resources
Full Text Sources

