Glycoconjugations of Biomolecules by Chemical Methods
- PMID: 33330359
- PMCID: PMC7672192
- DOI: 10.3389/fchem.2020.570185
Glycoconjugations of Biomolecules by Chemical Methods
Abstract
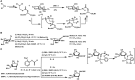
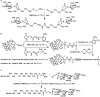
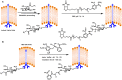
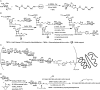
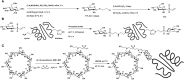
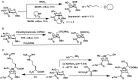
Bioconjugations under benign aqueous conditions have the most promise to covalently link carbohydrates onto chosen molecular and macromolecular scaffolds. Chemical methodologies relying on C-C and C-heteroatom bond formations are the methods of choice, coupled with the reaction conditions being under aqueous milieu. A number of methods, including metal-mediated, as well as metal-free azide-alkyne cyclo-addition, photocatalyzed thiol-ene reaction, amidation, reductive amination, disulfide bond formation, conjugate addition, nucleophilic addition to vinyl sulfones and vinyl sulfoxides, native chemical ligation, Staudinger ligation, olefin metathesis, and Suzuki-Miyaura cross coupling reactions have been developed, in efforts to conduct glycoconjugation of chosen molecular and biomolecular structures. Within these, many methods require pre-functionalization of the scaffolds, whereas methods that do not require such pre-functionalization continue to be few and far between. The compilation covers synthetic methodology development for carbohydrate conjugation onto biomolecular and biomacromolecular scaffolds. The importance of such glycoconjugations on the functional properties is also covered.
Keywords: biomolecules; carbohydrates; conjugations; glycoconjugations; lipids; nucleic acids; peptides; proteins.
Copyright © 2020 Sarkar and Jayaraman.
Figures

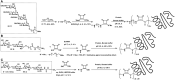
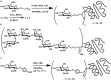
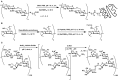

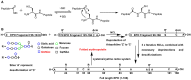

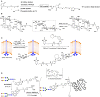

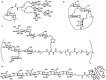

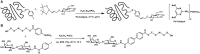
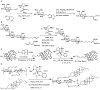


References
-
- Aljarilla A., López J. C., Plumet J. (2010). Metathesis reactions of carbohydrates: recent highlights in cross-metathesis. Eur. J. Org. Chem. 2010, 6123–6143. 10.1002/ejoc.201000570 - DOI
-
- Allen J. R., Allen J. G., Zhang X.-F., Williams L. J., Zatorski A., Ragupathi G., et al. . (2000). A second generation synthesis of the MBr1 (globo-H) breast tumor antigen: new application of the n-pentenyl glycoside method for achieving complex carbohydrate protein linkages. Chem. Eur. J. 6, 1366–1375. 10.1002/SICI1521-3765200004176:8<1366::AID-CHEM1366>3.0.CO - DOI - PubMed
-
- Arakatsu Y., Ashwell G., Kabat E. A. (1966). Immunological studies on dextrans. V. Specificity and cross reactivity with dextrans of the antibodies formed in rabbits to isomaltonic and isomaltotrionic acids coupled to serum albumin. J. Immunol. 97, 858–866. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

