Cobalt-Based Metal-Organic Frameworks and Their Derivatives for Hydrogen Evolution Reaction
- PMID: 33330381
- PMCID: PMC7715014
- DOI: 10.3389/fchem.2020.592915
Cobalt-Based Metal-Organic Frameworks and Their Derivatives for Hydrogen Evolution Reaction
Abstract
Hydrogen has been considered as a promising alternative energy to replace fossil fuels. Electrochemical water splitting, as a green and renewable method for hydrogen production, has been drawing more and more attention. In order to improve hydrogen production efficiency and lower energy consumption, efficient catalysts are required to drive the hydrogen evolution reaction (HER). Cobalt (Co)-based metal-organic frameworks (MOFs) are porous materials with tunable structure, adjustable pores and large specific surface areas, which has attracted great attention in the field of electrocatalysis. In this review, we focus on the recent progress of Co-based metal-organic frameworks and their derivatives, including their compositions, morphologies, architectures and electrochemical performances. The challenges and development prospects related to Co-based metal-organic frameworks as HER electrocatalysts are also discussed, which might provide some insight in electrochemical water splitting for future development.
Keywords: cobalt-base catalysts; electrocatalysts; hydrogen evolution reaction; metal-organic frameworks; water electrolysis.
Copyright © 2020 Han, Li, Ma and Yang.
Figures




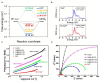
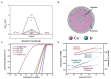
References
-
- Ahn W., Park M. G., Lee D. U., Seo M. H., Jiang G., Cano Z. P., et al. (2018). Hollow multivoid nanocuboids derived from ternary Ni–Co–Fe prussian blue analog for dual-electrocatalysis of oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 28:1802129 10.1002/adfm.201802129 - DOI
-
- Ao K., Dong J., Fan C., Wang D., Cai Y., Li D., et al. (2018). Formation of yolk–shelled nickel–cobalt selenide dodecahedral nanocages from metal–organic frameworks for efficient hydrogen and oxygen evolution. ACS Sustain. Chem. Eng. 6, 10952–10959. 10.1021/acssuschemeng.8b02343 - DOI
-
- Chen J., Liu J., Xie J.-Q., Ye H., Fu X.-Z., Sun R., et al. (2019). Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range. Nano Energy 56, 225–233. 10.1016/j.nanoen.2018.11.051 - DOI
Publication types
LinkOut - more resources
Full Text Sources

