Proteoglycans as Mediators of Cancer Tissue Mechanics
- PMID: 33330449
- PMCID: PMC7734320
- DOI: 10.3389/fcell.2020.569377
Proteoglycans as Mediators of Cancer Tissue Mechanics
Abstract
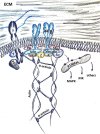
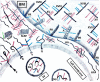
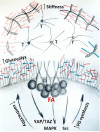
Proteoglycans are a diverse group of molecules which are characterized by a central protein backbone that is decorated with a variety of linear sulfated glycosaminoglycan side chains. Proteoglycans contribute significantly to the biochemical and mechanical properties of the interstitial extracellular matrix where they modulate cellular behavior by engaging transmembrane receptors. Proteoglycans also comprise a major component of the cellular glycocalyx to influence transmembrane receptor structure/function and mechanosignaling. Through their ability to initiate biochemical and mechanosignaling in cells, proteoglycans elicit profound effects on proliferation, adhesion and migration. Pathologies including cancer and cardiovascular disease are characterized by perturbed expression of proteoglycans where they compromise cell and tissue behavior by stiffening the extracellular matrix and increasing the bulkiness of the glycocalyx. Increasing evidence indicates that a bulky glycocalyx and proteoglycan-enriched extracellular matrix promote malignant transformation, increase cancer aggression and alter anti-tumor therapy response. In this review, we focus on the contribution of proteoglycans to mechanobiology in the context of normal and transformed tissues. We discuss the significance of proteoglycans for therapy response, and the current experimental strategies that target proteoglycans to sensitize cancer cells to treatment.
Keywords: GAG; cancer; glycocalyx; mechanosignaling; proteoglycans.
Copyright © 2020 Barkovskaya, Buffone, Žídek and Weaver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adler M., Larocca L., Trovato F. M., Marcinkowski H., Pasha Y., Taylor-Robinson S. D. (2016). Evaluating the risk of hepatocellular carcinoma in patients with prominently elevated liver stiffness measurements by FibroScan: a multicentre study. HPB 18 678–683. 10.1016/j.hpb.2016.05.005 - DOI - PMC - PubMed
-
- Ahmadzadeh H., Webster M. R., Behera R., Jimenez Valencia A. M., Wirtz D., Weeraratna A. T., et al. (2017). Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc. Natl. Acad. Sci. U. S. A. 114 E1617–E1626. 10.1073/pnas.1617037114 - DOI - PMC - PubMed
-
- Alberton P., Dugonitsch H. C., Hartmann B., Li P., Farkas Z., Saller M. M., et al. (2019). Aggrecan hypomorphism compromises articular cartilage biomechanical properties and is associated with increased incidence of spontaneous osteoarthritis. Int. J. Mol. Sci. 20:1008. 10.3390/ijms20051008 - DOI - PMC - PubMed

