Roles of Aminoacyl-tRNA Synthetases in Cancer
- PMID: 33330488
- PMCID: PMC7729087
- DOI: 10.3389/fcell.2020.599765
Roles of Aminoacyl-tRNA Synthetases in Cancer
Abstract
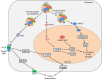
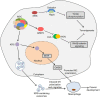
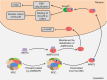
Aminoacyl-tRNA synthetases (ARSs) catalyze the ligation of amino acids to their cognate transfer RNAs (tRNAs), thus playing an important role in protein synthesis. In eukaryotic cells, these enzymes exist in free form or in the form of multi-tRNA synthetase complex (MSC). The latter contains nine cytoplasmic ARSs and three ARS-interacting multifunctional proteins (AIMPs). Normally, ARSs and AIMPs are regarded as housekeeping molecules without additional functions. However, a growing number of studies indicate that ARSs are involved in a variety of physiological and pathological processes, especially tumorigenesis. Here, we introduce the roles of ARSs and AIMPs in certain cancers, such as colon cancer, lung cancer, breast cancer, gastric cancer and pancreatic cancer. Furthermore, we particularly focus on their potential clinical applications in cancer, aiming at providing new insights into the pathogenesis and treatment of cancer.
Keywords: ARS-interacting multifunctional protein; aminoacyl-tRNA synthetase; cancer; pathogenesis; therapeutics.
Copyright © 2020 Zhou, Sun, Nie, Yu and Bian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comprehensive data resources and analytical tools for pathological association of aminoacyl tRNA synthetases with cancer.Database (Oxford). 2015 Mar 29;2015:bav022. doi: 10.1093/database/bav022. Print 2015. Database (Oxford). 2015. PMID: 25824651 Free PMC article.
-
Roles of aminoacyl-tRNA synthetase-interacting multi-functional proteins in physiology and cancer.Cell Death Dis. 2020 Jul 24;11(7):579. doi: 10.1038/s41419-020-02794-2. Cell Death Dis. 2020. PMID: 32709848 Free PMC article. Review.
-
The pathophyiological role of aminoacyl-tRNA synthetases in digestive system diseases.Front Physiol. 2022 Aug 9;13:935576. doi: 10.3389/fphys.2022.935576. eCollection 2022. Front Physiol. 2022. PMID: 36017335 Free PMC article. Review.
-
Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers.Trends Biochem Sci. 2005 Oct;30(10):569-74. doi: 10.1016/j.tibs.2005.08.004. Trends Biochem Sci. 2005. PMID: 16125937 Review.
-
Structure and Dynamics of the Human Multi-tRNA Synthetase Complex.Subcell Biochem. 2022;99:199-233. doi: 10.1007/978-3-031-00793-4_6. Subcell Biochem. 2022. PMID: 36151377
Cited by
-
The Aminoacyl-tRNA Synthetase and tRNA Expression Levels Are Deregulated in Cancer and Correlate Independently with Patient Survival.Curr Issues Mol Biol. 2022 Jul 2;44(7):3001-3017. doi: 10.3390/cimb44070207. Curr Issues Mol Biol. 2022. PMID: 35877431 Free PMC article.
-
Towards Understanding COVID-19: Molecular Insights, Co-infections, Associated Disorders, and Aging.J Alzheimers Dis Rep. 2021 Jul 20;5(1):571-600. doi: 10.3233/ADR-210010. eCollection 2021. J Alzheimers Dis Rep. 2021. PMID: 34514341 Free PMC article.
-
Construction of a prognostic model for lung adenocarcinoma based on membrane-tension-related genes.J Thorac Dis. 2023 Apr 28;15(4):2098-2115. doi: 10.21037/jtd-23-396. Epub 2023 Apr 23. J Thorac Dis. 2023. PMID: 37197492 Free PMC article.
-
"Proteotranscriptomic analysis of advanced colorectal cancer patient derived organoids for drug sensitivity prediction".J Exp Clin Cancer Res. 2023 Jan 6;42(1):8. doi: 10.1186/s13046-022-02591-z. J Exp Clin Cancer Res. 2023. PMID: 36604765 Free PMC article.
-
Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus.Int J Mol Sci. 2025 Mar 23;26(7):2912. doi: 10.3390/ijms26072912. Int J Mol Sci. 2025. PMID: 40243525 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

