Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification
- PMID: 33330547
- PMCID: PMC7710911
- DOI: 10.3389/fmed.2020.587773
Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification
Abstract
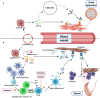
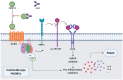
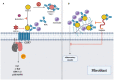
Systemic sclerosis (SSc) is an autoimmune disease characterized by abnormalities in microcirculation, extracellular matrix accumulation, and immune activation. Autoantibodies are markers of immune abnormalities and provide diagnostic and predictive value in SSc. Anti-topoisomerase antibodies (ATAs), anticentromere antibodies (ACAs), and anti-RNA polymerase antibodies (ARAs) are the three classical specific antibodies with the highest availability and stability. In this review, we provide an overview of the recent progress in SSc research with respect to ATAs, ACAs, and ARAs, focusing on their application in distinguishing clinical phenotypes, such as malignancy and organ involvement, identifying genetic background in human leukocyte antigen (HLA) or non-HLA alleles, and their potential roles in disease pathogenesis based on the effects of antigen-antibody binding. We finally summarized the novel analysis using ATAs, ACAs, and ARAs on more detailed disease clusters. Considering these advantages, this review emphasizes that classical SSc-specific autoantibodies are still practical and have the potential for patient and risk stratification with applications in precise medicine for SSc.
Keywords: anti-RNA polymerase antibodies; anti-topoisomerase antibodies; anticentromere antibodies; clinical manifestations; disease stratification; gene; systemic sclerosis.
Copyright © 2020 Yang, Tang, Zhu, Ding and Qiao.
Figures
References
-
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. (2013) 65:2737–47. 10.1002/art.38098 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

