This is a preprint.
Persistent Cellular Immunity to SARS-CoV-2 Infection
- PMID: 33330867
- PMCID: PMC7743071
- DOI: 10.1101/2020.12.08.416636
Persistent Cellular Immunity to SARS-CoV-2 Infection
Update in
-
Persistent cellular immunity to SARS-CoV-2 infection.J Exp Med. 2021 Apr 5;218(4):e20202515. doi: 10.1084/jem.20202515. J Exp Med. 2021. PMID: 33533915 Free PMC article.
Abstract
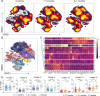
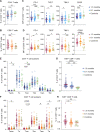
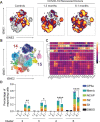
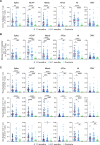
SARS-CoV-2 is responsible for an ongoing pandemic that affected millions of individuals around the globe. To gain further understanding of the immune response in recovered individuals we measured T cell responses in paired samples obtained an average of 1.3 and 6.1 months after infection from 41 individuals. The data indicate that recovered individuals show persistent polyfunctional SARS-CoV-2 antigen specific memory that could contribute to rapid recall responses. In addition, recovered individuals show enduring immune alterations in relative numbers of CD4 + and CD8 + T cells, expression of activation/exhaustion markers, and cell division.
Summary: We show that SARS-CoV-2 infection elicits broadly reactive and highly functional memory T cell responses that persist 6 months after infection. In addition, recovered individuals show enduring immune alterations in CD4 + and CD8 + T cells compartments.
Conflict of interest statement
Figures
References
-
- BRAUN J., LOYAL L., FRENTSCH M., WENDISCH D., GEORG P., KURTH F., HIPPENSTIEL S., DINGELDEY M., KRUSE B., FAUCHERE F., BAYSAL E., MANGOLD M., HENZE L., LAUSTER R., MALL M. A., BEYER K., ROHMEL J., VOIGT S., SCHMITZ J., MILTENYI S., DEMUTH I., MULLER M. A., HOCKE A., WITZENRATH M., SUTTORP N., KERN F., REIMER U., WENSCHUH H., DROSTEN C., CORMAN V. M., GIESECKE-THIEL C., SANDER L. E. & THIEL A. 2020. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature, 587, 270–274. - PubMed
-
- BRETON G., CHOMONT N., TAKATA H., FROMENTIN R., AHLERS J., FILALI-MOUHIM A., RIOU C., BOULASSEL M. R., ROUTY J. P., YASSINE-DIAB B. & SEKALY R. P. 2013. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol, 191, 2194–204. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
