This is a preprint.
Thiol drugs decrease SARS-CoV-2 lung injury in vivo and disrupt SARS-CoV-2 spike complex binding to ACE2 in vitro
- PMID: 33330868
- PMCID: PMC7743076
- DOI: 10.1101/2020.12.08.415505
Thiol drugs decrease SARS-CoV-2 lung injury in vivo and disrupt SARS-CoV-2 spike complex binding to ACE2 in vitro
Abstract
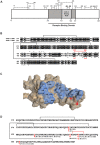
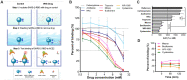
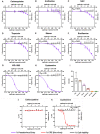
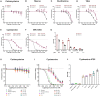
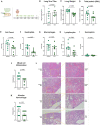
Neutrophil-induced oxidative stress is a mechanism of lung injury in COVID-19, and drugs with a functional thiol group ("thiol drugs"), especially cysteamine, have anti-oxidant and anti-inflammatory properties that could limit this injury. Thiol drugs may also alter the redox status of the cysteine-rich SARS-CoV-2 spike glycoprotein (SARS-2-S) and thereby disrupt ACE2 binding. Using ACE2 binding assay, reporter virus pseudotyped with SARS-CoV-2 spikes (ancestral and variants) and authentic SARS-CoV-2 (Wuhan-1), we find that multiple thiol drugs inhibit SARS-2-S binding to ACE2 and virus entry into cells. Pseudoviruses carrying variant spikes were less efficiently inhibited as compared to pseudotypes bearing an ancestral spike, but the most potent drugs still inhibited the Delta variant in the low millimolar range. IC50 values followed the order of their cystine cleavage rates and lower thiol pKa values. In hamsters infected with SARS-CoV-2, intraperitoneal (IP) cysteamine decreased neutrophilic inflammation and alveolar hemorrhage in the lungs but did not decrease viral infection, most likely because IP delivery could not achieve millimolar concentrations in the airways. These data show that thiol drugs inhibit SARS-CoV-2 infection in vitro and reduce SARS-CoV-2-related lung injury in vivo and provide strong rationale for trials of systemically delivered thiol drugs as COVID-19 treatments. We propose that antiviral effects of thiol drugs in vivo will require delivery directly to the airways to ensure millimolar drug concentrations and that thiol drugs with lower thiol pKa values are most likely to be effective.
Conflict of interest statement
Competing interests: John Fahy, Stefan Oscarson, Irina Gitlin and Wilfred Raymond are inventors on patent applications related to use of thiol-based drugs as treatments for COVID19. The other authors have no competing interests.
Figures

References
-
- Horby P., Lim W. S., Emberson J. R., Mafham M., Bell J. L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L. C., Faust S. N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J. K., Haynes R., Landray M. J., Dexamethasone in Hospitalized Patients with Covid-19, N. Engl. J. Med. 384, 693–704 (2021). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
