This is a preprint.
Model-informed COVID-19 vaccine prioritization strategies by age and serostatus
- PMID: 33330882
- PMCID: PMC7743091
- DOI: 10.1101/2020.09.08.20190629
Model-informed COVID-19 vaccine prioritization strategies by age and serostatus
Update in
-
Model-informed COVID-19 vaccine prioritization strategies by age and serostatus.Science. 2021 Feb 26;371(6532):916-921. doi: 10.1126/science.abe6959. Epub 2021 Jan 21. Science. 2021. PMID: 33479118 Free PMC article.
Abstract
Limited initial supply of SARS-CoV-2 vaccine raises the question of how to prioritize available doses. Here, we used a mathematical model to compare five age-stratified prioritization strategies. A highly effective transmission-blocking vaccine prioritized to adults ages 20-49 years minimized cumulative incidence, but mortality and years of life lost were minimized in most scenarios when the vaccine was prioritized to adults over 60 years old. Use of individual-level serological tests to redirect doses to seronegative individuals improved the marginal impact of each dose while potentially reducing existing inequities in COVID-19 impact. While maximum impact prioritization strategies were broadly consistent across countries, transmission rates, vaccination rollout speeds, and estimates of naturally acquired immunity, this framework can be used to compare impacts of prioritization strategies across contexts.
Conflict of interest statement
Competing Interests: ML discloses honoraria/consulting from Merck, Affinivax, Sanofi-Pasteur, Bristol Myers Squibb, and Antigen Discovery; research funding (institutional) from Pfizer; an unpaid scientific advice to Janssen, Astra-Zeneca, and Covaxx (United Biomedical); and is an Honorary Faculty Member, Wellcome Sanger Institute, and an Associate Member, Broad Institute. YHG discloses consulting for Merck and GlaxoSmithKline, and research funding from Pfizer not related to this project or topic. DBL is a member of the scientific advisory board of Darwin BioSciences.
Figures


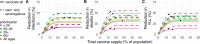
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. Online, 2020. (accessed January 4, 2021). https://coronavirus.jhu.edu/map.html.
-
- Khamsi Roxanne. If a coronavirus vaccines arrives, can the world make enough? Nature, 580(7805):578–580, April 2020. - PubMed
-
- Framework for equitable allocation of COVID-19 vaccine. Online, 2020. (accessed December 6, 2020). https://www.nap.edu/catalog/25917/framework-for-equitable-allocation-of-.... - PubMed
-
- Weycker Derek, Edelsberg John, Elizabeth Halloran M., Longini Ira M., Nizam Azhar, Ciuryla Vincent, and Oster Gerry. Population-wide benefits of routine vaccination of children against influenza. Vaccine, 23(10):1284 – 1293, 2005. - PubMed
-
- Medlock Jan and Galvani Alison P.. Optimizing influenza vaccine distribution. Science, 325(5948):1705–1708, September 2009. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
