This is a preprint.
Diverse Functional Autoantibodies in Patients with COVID-19
- PMID: 33330894
- PMCID: PMC7743105
- DOI: 10.1101/2020.12.10.20247205
Diverse Functional Autoantibodies in Patients with COVID-19
Update in
-
Diverse functional autoantibodies in patients with COVID-19.Nature. 2021 Jul;595(7866):283-288. doi: 10.1038/s41586-021-03631-y. Epub 2021 May 19. Nature. 2021. PMID: 34010947
Abstract
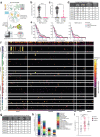
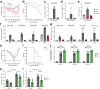
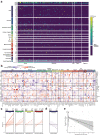
COVID-19 manifests with a wide spectrum of clinical phenotypes that are characterized by exaggerated and misdirected host immune responses1-8. While pathological innate immune activation is well documented in severe disease1, the impact of autoantibodies on disease progression is less defined. Here, we used a high-throughput autoantibody discovery technique called Rapid Extracellular Antigen Profiling (REAP) to screen a cohort of 194 SARS-CoV-2 infected COVID-19 patients and healthcare workers for autoantibodies against 2,770 extracellular and secreted proteins (the "exoproteome"). We found that COVID-19 patients exhibit dramatic increases in autoantibody reactivities compared to uninfected controls, with a high prevalence of autoantibodies against immunomodulatory proteins including cytokines, chemokines, complement components, and cell surface proteins. We established that these autoantibodies perturb immune function and impair virological control by inhibiting immunoreceptor signaling and by altering peripheral immune cell composition, and found that murine surrogates of these autoantibodies exacerbate disease severity in a mouse model of SARS-CoV-2 infection. Analysis of autoantibodies against tissue-associated antigens revealed associations with specific clinical characteristics and disease severity. In summary, these findings implicate a pathological role for exoproteome-directed autoantibodies in COVID-19 with diverse impacts on immune functionality and associations with clinical outcomes.
Conflict of interest statement
Competing Interests A.M.R., E.Y.W., and Y.D. are inventors of a patent describing the REAP technology.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
